More Information
Submitted: February 27, 2023 | Approved: March 14, 2023 | Published: March 15, 2023
How to cite this article: Stąsiek J, Jewartowski M, Baranski J, Wajs J. Modeling of low calorific gas burning in a deficient oxygen environment and high-temperature oxidizer. Int J Phys Res Appl. 2023; 6: 027-034.
DOI: 10.29328/journal.ijpra.1001050
Copyright License: © 2023 Stąsiek J, et al. This is an open access article distributed under the Creative Commons Attribution License, which peRmits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Modeling of low calorific gas burning in a deficient oxygen environment and high-temperature oxidizer
Jan Stąsiek*, Marcin Jewartowski, Jacek Baranski and Jan Wajs
The Gdansk University of Technology, Faculty of Mechanical Engineering and Ship Technology, Poland
*Address for Correspondence: Dr. Jan Stąsiek, MSc, MEng, PhD, Hab, DSc, Professor, Gdansk University of Technology, Faculty of Mechanical Engineering and Ship Technology, Institute of Energy, G. Narutowicza 11/12, 80-233 Gdansk, Poland, Email: [email protected]
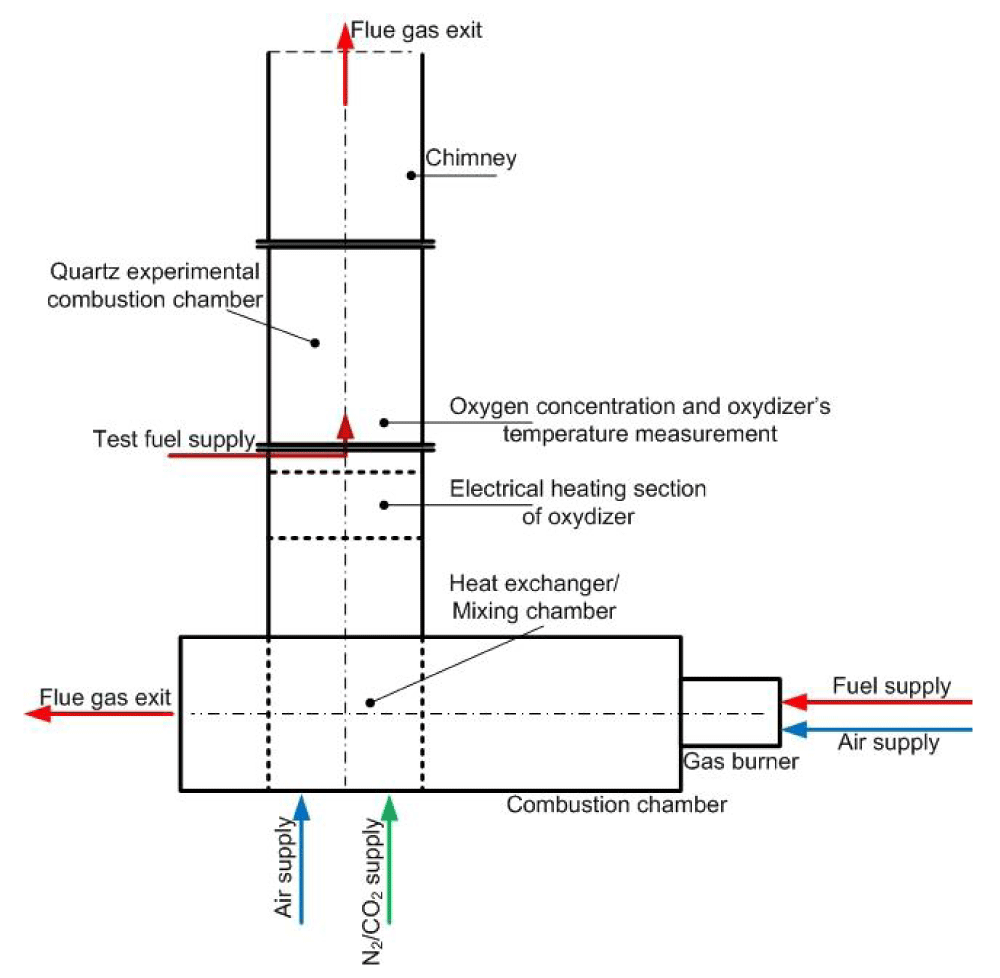
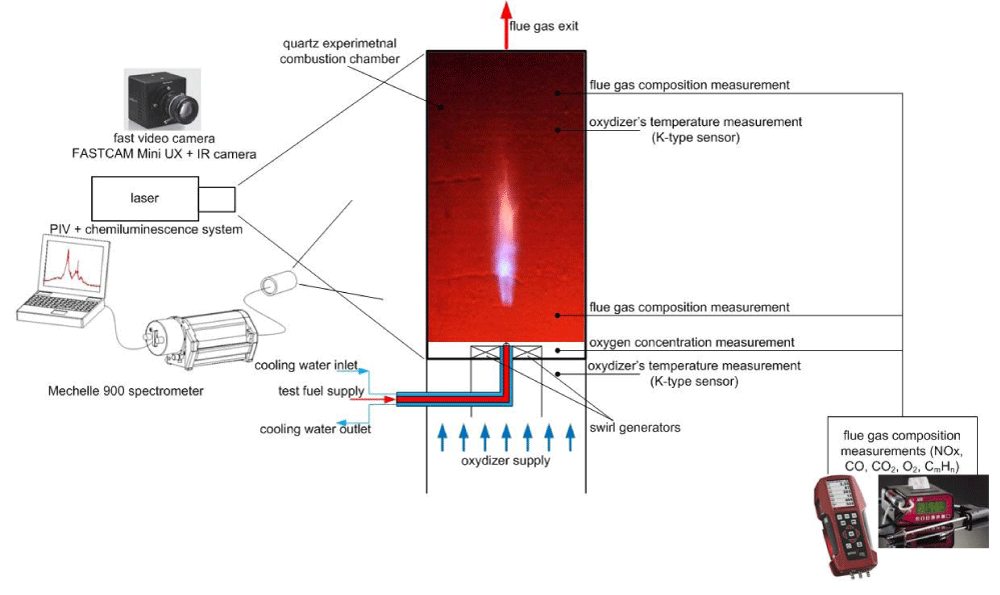
It is planned to carry out a comprehensive experimental and theoretical study on the high temperature of low calorific gas combustion with oxygen-deficient oxidizers. The experimental research will be performed using the experimental facility with a combustion chamber. The oxygen concentration in combustion oxidizers will be varied from 21% by volume (normal) air to 2%. The test combustion chamber will be fed with propane or methane as the reference fuel, then with low calorific fuels as test gases obtained by mixing various combustible components, e.g. H2, CH4, CO, and neutral gases, e.g. N2, CO2. Gaseous fuels prepared in this way will be burned in the atmosphere of a deficient oxidizer with a temperature changing from 800 °C to 1100 °C. Oxidizers will be heated up to a certain temperature using two methods: by flue gas heat exchanger and kanthal rod electric preheater. Different burner geometry will be used. The burner will be equipped with annular swirl vanes for co-axial or under different angles, fuel, and oxidizers flow to have a high swirl number achieved by flow aerodynamics and mixing. Experimental data will be verified with numerical simulations with the use of ANSYS CFD Fluent code.
The most recent combustion technology of fossil fuels provides the vast majority of the world’s energy consumption and is thus also a major source of air pollutant emissions of greenhouse gases. Nowadays several different combustion technologies have been developed to meet new and more stringent demands on energy consumption, environmental impact, and production expenditure.
Therefore, the general objective of the proposed project is to increase the understanding of the low calorific fuel (for example coming from thermal conversion of biomass and wastes) combustion process under high temperature and low oxygen oxidizer. These kinds of conditions are representative of flameless oxidation (FLOX) also known as high-temperature air combustion (HiTAC or HTAC), intense low oxygen dilution (MILD) combustion, or colorless distributed combustion (CDC). This technology offers good potential for stable combustor operation, nearly zero pollutants emission, noise reduction, fuel flexibility, and compact size of devices. Detailed knowledge of distributed combustion behavior (especially in low calorific fuel) is required to further develop this technology. The entrainment of hot oxygen-deficient oxidizers will be simulated by diluting the inlet air stream with inert nitrogen or carbon dioxide and heating it to a high temperature. It is planned to seek the best configuration of combustion test rig and burner equipped with a swirler, to make it favorable for many practical combustor applications and burners designed. All these activities will be combined with the best possible temperature profiles and flow visualization, dedicated to recognizing the mechanisms of such technology and possible accompanying phenomena.
State-of-the-art (evolution of flame research under HiTAC technology)
MILD combustion is defined as: ‘A combustion process is named MILD when the inlet temperature of the reactant mixture is higher than mixture self-ignition temperature whereas the maximum allowable temperature increase with respect to inlet temperature during combustion is lower than mixture self-ignition temperature (in Kelvin)’. This definition shares many similarities with that of HiTAC, as the temperature in the latter can be controlled through the transfer of heat and mass of hot product gases into the inlet reactant stream. The mixture self-ignition temperature of a well-stirred reactor is defined as the inlet temperature of which any incremental temperature increase causes the system to reach a state where the chemical process is self-sustaining. Furthermore, MILD combustion was concluded to differ from other combustion regimes in that the evolution of the latter is characterized by a wide temperature range where kinetics and physical parameters may change abruptly or chaotically during the process. In MILD combustion, a gradual evolution is achieved. Except for the requirement of a maximum temperature increase in MILD combustion, which will be inherently fulfilled in most if not all cases, it is seen that the definitions of HiTAC and MILD combustion are nearly identical. Similar can be said about flameless oxidation FLOX, which got its name from the lack of visible flame during experiments with highly preheated air, even though all the fuel was burned. However, Weber, et al. [1] insist, that FLOX should be distinguished from HiTAC. FLOX is described as technology where fuel is injected through the central jet while the combustion air, preheated using a recuperator or a regenerator, is injected by a number of small nozzles located on a circumference separated from the fuel jet. For gaseous fuels free of fuel nitrogen, the NOx emissions of FLOX burners are as low as those from the HiTAC technology, however, for nitrogen-containing fuels, FLOX technology is not as effective and efficient as HiTAC. No matter what it is called, they all rely on the same basic principles: dilution of reactants prior to combustion by recirculation of exhaust gases at high turbulence levels and with the use of preheated combustion air. In CDC oxidation processes are associated with a low Damköhler (Da) number (the ratio of flow and chemical time scales) and thus physical processes may no longer be the rate-limiting step. The Damköhlernumber is defined as the ratio of the flow time scale (defined by the ratio of the integral length scale and root-mean-square velocity fluctuation) to the chemical time scale (defined by the ratio of flame thickness and flame speed). The HiTAC and CDC flame regimes are characterized by low Damköhler numbers in relation to turbulent Reynolds numbers. A sufficiently low Damköhler number is achieved by increasing the chemical time scale or lowering the flow time scale. It was found that lowering the oxygen concentration significantly increases flame thickness while reducing the flame speed and thus increases the chemical time scale. Distributed combustion can be achieved solely through air dilution by increasing the air jet mass flow rate. However, pure air dilution lowers the Damköhler number to a smaller extent while also increasing the turbulent Reynolds number, forcing the flame closer to the ’flamelets in eddies’ regime.
Systematic studies of combustion in highly preheated and low oxygen content oxidizers started about two decades ago. Hasegawa, et al. [2] performed a study with the NKF test facility, designed and fabricated by the Nippon Furnace Kogyo Kaisha, NFK, and consisting of two combustion chambers, each fitted with ceramic honeycomb regenerator, controllers for flow and switching sequence. The high-temperature combustion air, produced from the regenerative test facility, and a gas jet can then be injected into this preheated oxidizer. In their work, a mixture of air, N2, and CO2 was used as the oxidizer. They reported that the single-jet gas flame flammability does not decrease with decreasing in oxygen concentration when the oxidizer temperature is sufficiently high. For example, the limiting temperature was 900 °C for the LPG fuel. They also found that the HiTAC flame had much better uniformity of temperature and the temperature fluctuations went from 197 °C under the conventional conditions down to below 5 °C for HiTAC. They also reported that the flame with 3% O2 in the oxidizer and preheated to 1010 °C had very low visible radiation and a much larger volume. The color of the flames was reported to be blue at conventional conditions and ‘Blueish-green’ or green at HiTAC firings. They found that CO2 had a stronger effect on NOx reduction than if N2 was used to dilute the oxidizer.
Kishimoto, et al. [3] performed experiments on a similar NFK HTAC test facility. Natural gas was chosen as fuel, and N2 as dilutant and they took chemiluminescence images. They found that the flame seemed to be stabilized at a large distance from the injection nozzle. When diluting the oxidizer that the dilution made the flame unstable, increasing the temperature made the flame stable again. They reported that the flame color changed from violet to blue and then to green. Their explanation was the decreasing CH emission and the increasing C2 emission.
Amagai, et al. [4] made an experiment in steady-state with an electric heat oxidizer into which they injected a co-flowing single fuel jet of propane. Both gas jet diffusion and premixed flames were checked. The results reported that both the diffusion and premixed flame were well stabilized when the temperature was over the auto-ignition temperature. This work also demonstrated that the flame length of the laminar diffusion and the premixed flames decrease with increasing temperature of the oxidizer temperature. On the other hand, the length of turbulent diffusion increases with flame-laminarization due to high temperature.
Kitagawa, et al. [5] used a spectra-video camera to capture the fluctuations of LPG flame in an oxidizer diluted with N2 from the NFK HTAC test facility. They also measured the temperature profile in the combustion chamber with the thermocouple. They calculated the vibration temperature in 2-D images of the flame using the two C2 bands. A very uniform temperature profile was found at high preheat and low oxygen concentrations, which coincided with the lowest temperature fluctuations.
Isiguro, et al. [6] have studied uniformity and homogenization of combustion of methane and propane with highly preheated air (1000 °C). They obtained images of OH, CH, and C2 emissions for a number of experimental conditions that differed in the air preheat level and oxygen content of the air. The investigators concluded that the increase in air temperature results in a decrease in flame temperature gradients (homogenization of flames). Furthermore, they concluded, that the higher the combustion air temperature the lower the flame fluctuations.
Plessing, et al. [7] used laser-induced pre-dissociative fluorescence and Rayleigh thermometry to examine flameless oxidation at a laboratory scale. They observed that flameless oxidation occurs in the well-stirred reactor regime. The OH concentration in the combustion zones of flameless oxidation is lower than in non-preheated undiluted turbulent premixed flames. Soon afterward studies have been undertaken on the effect of combustion air temperature and oxygen concentration on flame color, visibility and thermal emission spectra. Both methane and propane were used as fuel. The spectroscopy measurements checked that there were large emissions of CH and C2 as well as other intermediate species for propane flame. But in the methane flame, they did not measure such significant emissions. However, they noticed that there were much fewer intermediate species emissions for the methane flame.
Gupta [8] and Bolz [9] further investigated the influences on HiTAC volume and lift-off (standoff) distances with direct flame photography. In this work, N2 was a diluted oxidizer and the fuel was LPG. They found that the flame color varied from yellow, at 21% O2 and 1100 °C, to a yellow-blue flame, when decreasing the oxygen concentration. And at the HiTAC firing, the flame was found to be blue-green. At 900 °C, however, the flame changed from yellow to blue, without any green color. Gupta, et al. [10] further investigations found no yellow color in flames with less than 950 °C and low oxygen concentration in the oxidizer. They concluded that the effect of oxygen concentration and oxidizer temperature on flame color be of use when designing applications that need certain radiant heat flux.
Mochida and Hasegawa [11] have been developing a flame visualization technique based on the luminescence intensity ratio of C2 and CH radicals.
Lille, et al. [12] built an experimental facility for studying fuel jets immersing into a cross-flowing high-temperature air stream and their preliminary findings have been reported. The fuel was propane and N2 was used as diluting. High-speed photography, and direct and schlieren color visualization were used to record images of flames. They conducted that a lower oxygen concentration increases the flame size. Air temperature has the opposite effect of decreasing the flame size but not in the same proportions as an increase in oxygen content. The flame visibility decreases with decreasing oxygen concentration and increases with air temperature. The flame color changes from bright white/yellow to blue/green/yellow and is primarily influenced by oxygen concentration. Lille’s work depicted that a lower oxygen concentration increases the lift-off distance, and for high-velocity fuel, the jet increases the lift-off distance. Lille, et al. [13] further investigated fuel jet combustion under the condition of high temperatures and oxygen-deficient air. In this work, the jet of fuel was co-axially injected into high-temperature exhaust gases generated by means of a gas burner fired with Gasol (mainly propane). They found that the oxygen concentration in the oxidizer has a substantial effect on flame size, luminosity, color, and visibility. The flame became first bluish and then non-visible at sufficiently low oxygen concentrations in the oxidizer, Figures 1,2.
Figure 1: Line of internal force.
Figure 2: Combustion chamber with measurement equipment.
In the co-flow study of Fujimori, et al. [14] and Sato, et al. [15] it was demonstrated that the reduction of NOx emission was largest for flames with high liftoff distance, and diluting the fuel with N2 had a considerable effect on the NOx reduction. The same results were found for air dilution. Blasiak, et al. [16] reported that the addition of N2 to methane results in lower emission of NO and CO, as well as higher fuel injection velocity.
Rota, et al. [17] presented an experiment furnace in which partially premised fuel was co-axially injected into a preheated and diluted air stream. The investigations gave that the NOx emission was decreased when the process run under HiTAC conditions.
Using the NFK HiTAC test stand, Mochida, et al. [18] investigated the flame radiation by means of a CCD camera. The results showed that the relative intensity of HiTAC flame is much lower, approximately one-third, and is longer than a conventional firing. They explained that the radical particles, which produce a more luminous flame, were formed in conventional firing compared to the HiTAC flame.
Numerical studies of HiTAC flame were conducted among others by Yuan, et al. [19], Dong [20], and Yang [21]. They showed the influence of dilution of air steam on maximum flame temperature and NOx emission and gave out reasonable predictions of NOx formation.
In the book on HiTAC [22], the characteristics of HiTAC combustion were analyzed. HTAC firing is controlled both by chemical kinetics and by mixing. Then, the mixed fraction/PDF model and Eddy-Break-Up model are not suitable for HiTAC, since the chemical reactions of both of them are assumed to be infinitely fast, furthermore, PDF model was experientially obtained based on normal combustion reaction, which implicitly infers the use of ambient air. Thus, the eddy dissipation concept with a multi-step reaction of fuel is an interesting suggestion for calculating HiTAC at present. Furthermore, the single gas jet combustion under the condition of high temperature and deficient air was carried out. The combustion models were investigated and compared. These models include the one-step global reaction model, Jones’s four-step reaction model, and Srivatsa’s four-step reaction model. The results show that Srivatsa’s model was better among the three models concerning the flame-lifted height and maximum flame temperature.
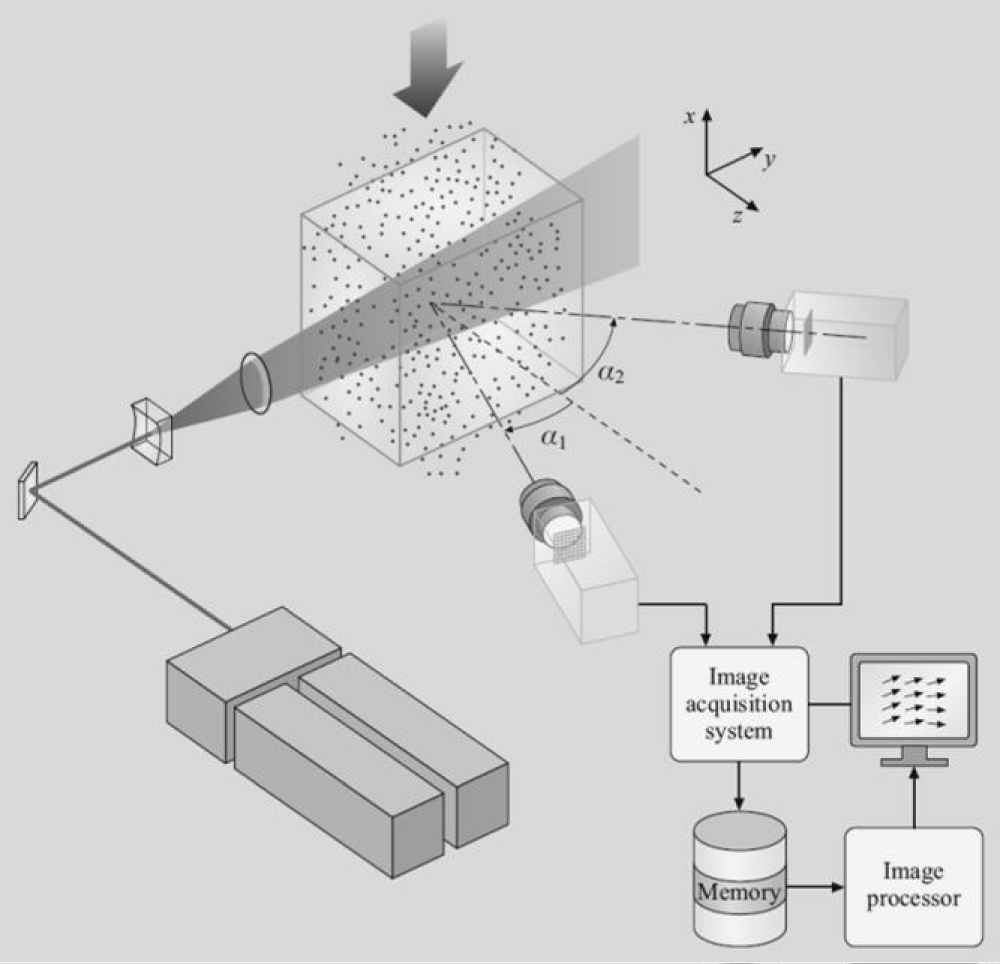
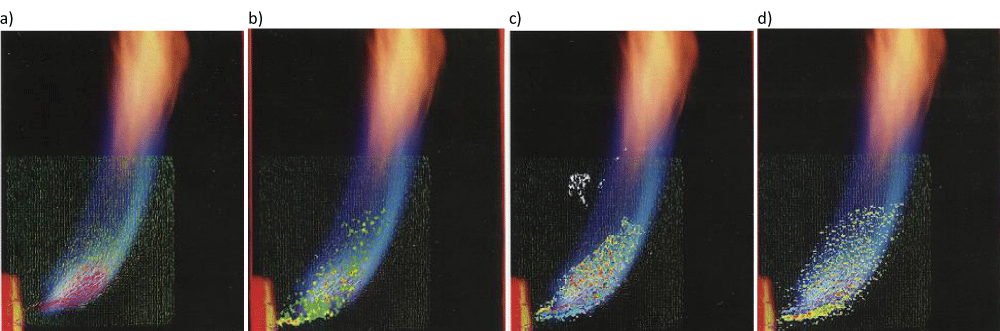
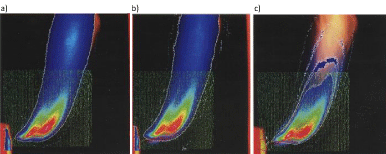
Mörtberg, et al. [23] used Particle Image Velocimetry (PIV) to obtain information on the flow dynamics of a fuel jet injected into a cross-flow of oxidizer at either a normal temperature or a very high temperature, Figure 3. Light emission spectroscopy was used to collect information on time-averaged radical distribution in the combustion jet. Jet turbulence, time-averaged velocity distribution, fuel jet mixing, the distribution of radicals such as CH, OH, and C2 and flame images were investigated, in Figures 4,5. The results showed delayed mixing and combustion under high-temperature low oxygen concentration conditions. The combustion air preheats temperature and oxygen concentrations were found to have a significant effect on the burning and fuel-jet behavior.
Figure 3: Schematic of a typical PIV measurement with two cameras and superimposed data of flame photograph of OH distributioninC3H8 jet flame at 1173K and 5% oxygen concentration.
Figure 4: Superimposed data of flame photograps: a) average velocity field, b) radial RMS values, C) vorticity distribution and d) axial strain rate distribution. The fuel jet was C3H8 at 1173 K and 5% oxygen concentration.
Figure 5: Superimposed data of flame photograps and light emission spectroscopy data: a)C2-distribution, b) CH- distribution, C) OH- distribution. The fuel jet was C3H8 at 1173 K and 5% oxygen concentration in the oxidizer.
The use of high-temperature and low oxygen-content air in regenerative burners in a semi-industrial furnace equipped with a radiant tube was investigated by Rafidi, et al. [24]. Significant improvement in temperature distribution along the tube as well as higher efficiency of the process was reported in comparison with conventional combustion with a recuperative burner. More recent investigations with the radiant tube can be found in [25].
Roy, et al. [26], Karyeyen, et al. [27] Khail, et al. [28] more recently presented results of flame structure and emission signature under colorless distributed combustion regime using different gaseous hydrocarbon fuels with a swirl-stabilized combustor. The flame lift-off heights were measured to explore the nature of different flames when approaching CDC conditions. The location and global structure of swirl-stabilized flame at different O2 concentrations corresponding to different flow dilution levels were determined from the OH chemiluminescence signature. Khail, et al. [29] investigated also the influence of fuel properties (including hydrogen-enriched fuels) on colorless distributed combustion. Single gas jet combustion under the condition of high-temperature and deficient air was carried out. The combustion models were investigated and compared. These models include the one-step global reaction model, Jones’s four-step reaction model, and Srivatsa’s four-step reaction model. The results show that Srivatsa’s model was better among the three models concerning the flame-lifted height and maximum flame temperature.
Mörtberg, et al. [23] used Particle Image Velocimetry (PIV) to obtain information on the flow dynamics of a fuel jet injected into a cross-flow of oxidizer at either a normal temperature or a very high temperature. Light emission spectroscopy was used to collect information on time-averaged radical distribution in the combustion jet. Jet turbulence, time-averaged velocity distribution, fuel jet mixing, the distribution of radicals such as CH, OH and C2, and flame images were investigated. The results showed delayed mixing and combustion under high-temperature low oxygen concentration conditions. The combustion air preheats temperature and oxygen concentrations were found to have a significant effect on the burning and fuel-jet behavior.
The use of high-temperature and low-oxygen-content air in regenerative burners in a semi-industrial furnace equipped with a radiant tube was investigated by Rafidi, et al. [24]. Significant improvement in temperature distribution along the tube as well as higher efficiency of the process was reported in comparison with conventional combustion with a recuperative burner. More recent investigations with the radiant tube can be found in [25].
Roy, et al. [26], Karyeyen, et al. [27] Khail, et al. [28] more recently presented results of flame structure and emission signature under colorless distributed combustion regime using different gaseous hydrocarbon fuels with a swirl-stabilized combustor. The flame lift-off heights were measured to explore the nature of different flames when approaching CDC conditions. The location and global structure of swirl-stabilized flame at different O2 concentrations corresponding to different flow dilution levels were determined from the OH chemiluminescence signature. Khail, et al. [29] investigated also the influence of fuel properties (including hydrogen-enriched fuels) on colorless distributed combustion.
Although HiTAC started with industrial furnaces, as stated by Weber, et al. [1] in a paper focusing on a historical review of HiTAC technology, other applications were also proposed and investigated by different researchers. The possible use of high-temperature air combustion in utility boilers was considered, among the others, by Schaffel-Mancini, et al. [30]. A few review articles presenting the possibility and potential of adaptation of HiTAC in gas turbines were given recently by Xing et al [31], Khidr, et al. [32], and Perpignan, et al. [33].
High-preheating air has been used with success also in gasification in technology called high-temperature air/steam gasification (HiTAG/HiTSG). Some of the results are presented in [34-36] and they show the favorable influence of high-temperature agents on gasification product quality.
Significance of the project
Modern technologies for thermal conversion of waste and low calorific fuels are expected to simultaneously meet the requirements in terms of reliability, economy, and environmental protection. During the past twenty years independent studies carried out on laboratory, pilot, and full-scale units suggest many distinct advantages of combustion with high-temperature low oxygen content oxidizers as compared to any other combustion method. The thermal and chemical behavior of flames thus formed is significantly different than that of normal air flames. The differences include increasing thermal field uniformity in the combustion zone leading to high efficiency and low fuel consumption. Further examination of the technology has revealed other advantages, such as very low emission of pollutants. Furthermore, low-heating value fuel can be utilized without any auxiliary fuel. The smaller size of devices for performing the same function means savings in materials and resources. This in turn means further energy savings and pollution reduction. This technology has created a significant impact on the design and development of advanced industrial furnaces, which were its first applications, and is very promising in the field of gas turbines and internal combustion engines. Detailed knowledge of this type of combustion behavior (especially for low calorific fuel) is required to further deploy this technology.
Concept and research work plan
It is planned to carry out a comprehensive experimental and theoretical study on the high temperature of low calorific gas combustion with oxygen-deficient oxidizers. The experimental research will be performed using the experimental facility with a combustion chamber. The oxygen concentration in combustion oxidizers will be varied from 21% by volume (normal) air to 2%. The test combustion chamber will be fed with propane or methane as the reference fuel, then with low calorific fuels as test gases obtained by mixing various combustible components, e.g. H2, CH4, CO and neutral gases, e.g. N2, CO2. Gaseous fuels prepared in this way will be burned in the atmosphere of deficient oxidizers with a temperature changing from 800 °C to 1100 °C. Oxidizers will be heated up to a certain temperature using two methods: by flue gas heat exchanger and kanthal rod electric preheater. Different burner geometry will be used. The burner will be equipped with annular swirl vanes for co-axial or under different angles, fuel, and oxidizers flow to have a high swirl number achieved by flow aerodynamics and mixing. Experimental data will be verified by 3D numerical simulations of low calorific gas fuels combustion process. The commercial ANSYS CFD Fluent code will be used. The software is a steady state/transient, finite volume program that can solve three-dimensional fields of pressure, velocity, temperature, the kinetic energy of turbulence, and the dissipation rate of turbulence and chemical species. The Fluent CFD software operates by solving the governing differential equations of the flow physics by numerical means on a computational mesh and can predict gas velocity, temperature profile, and concentration of free radicals, such as C2, CH and OH.
The combustion proceeds in an atmosphere of low oxygen concentration, as well as at high temperatures of the oxidizer, mostly above the auto-ignition temperature of the fuel, spread out many new features, such as significantly higher flame stability at all fuel-air (including very lean mixtures) a larger flame volume, uniform temperature distributions, higher heat transfer by radicals radiation (C2, OH, CH) and low NOx emission. These features have been demonstrated in many practice applications, either with air as oxidizers combing a modern regenerative system or with pure oxygen as oxidizers. Bearing in mind the demand of the energy and metallurgical industry for the combustion of low calorific gas in an oxygen-poor oxidant a comprehensive work will be provided, in regard to the fundamental differences in the thermal, chemical, and fluid dynamics characteristics of the flame developed by swirl –stabilized burner. However, since the high temperature and low oxygen deficiency are the unique parameters that differ from conventional combustion, there is also little knowledge of their quantitative effects of them on the flame properties. Detailed knowledge of distributed combustion behavior is required to further deploy this technology.
- Weber R, Gupta AK, Mochida S. High temperature air combustion (HiTAC): How it all started for applications in industrial furnaces and future prospects. Applied Energy. 2020; 278:115551.
- Hasegawa T, Tanaka R, Niioka T. The 1st Asia-Pacific Conference on Combustion. Osaka, Japan. 1997; 290-293.
- Kishimoto K, Watanabe Y, Kasahara M, Hasegawa T, Tanaka R. The 1st Asia-Pacific Conference on Combustion, Osaka, Japan. 1997; 468-471.
- Amagai K, Arai M. RAN95. Int. Symposium on Advanced Energy Conversion Systems and Related Technologies. Nagoya. 1995; 703-710.
- Kitagawa K, Konishi N, Arai N, Gupta AK. FACT-Vol.22, Int. Joint Power Generation Conference. ASME. 1998; 1: 239-242.
- Isiguro T, Tsuge S, Furuhata T, Kitagawa K, Aral N, Hasegawa T, Tanaka R. Gupta AK. Homogenization and stabilization during combustion of hydrocarbons with preheated air. Symposium (International) on Combustion. 1998; 27: 3205-3213.
- Plessing T, Peters N, Wunning JG. Laseroptical investigation of highly preheated combustion with strong exhaust gas recirculation. Symposium (International) on Combustion. 1998; 27: 3197-3204.
- Gupta AK, Li Z. IJPGC. ASME EC. 1997; 5.
- Bolz S, Gupta AK. FACT-Vol.22. Int Joint Power Generation Conference. ASME. 1998; 1: 193-205.
- Gupta AK, Bolz S, Hasegawa T. Effect of Air Preheat Temperature and Oxygen Concentration on Flame Structure and Emission. Journal of Energy Resources Technology. 1999; 121:209-216.
- Mochida S, Hasegawa T. Proceedings of the 2nd International Seminar on High-Temperature Combustion in Industrial Furnaces. Jernkontoret-KTH, Stockholm, Sweden. 2000.
- Lille S, Dobski T, Blasiak W. Visualization of Fuel Jet in Conditions of Highly Preheated Air Combustion. AIAA Journal of Propulsion and Power. 2000; 16(4): 595-600.
- Lille S, Blasiak W, Jewartowski M. Experimental study of the fuel jet combustion in high temperature and low oxygen content exhaust gases. Energy. 2005; 30( 2-4): 373-384.
- Fujimori T, Riechelmann D, Sato J. 1st ASPAC, Osaka, Japan, May 1997.
- Sato J. 1st ASPAC, Osaka, Japan, May 12-15, 1997.
- Blasiak W, Szewczyk D, Dobski T. Proceedings of IJPGC’01, paper FACT-19048, New Orleans, USA, 4-7 June 2001.
- Rota R, Guarneri F, Gelosa D, Effuggi A, Rabaioli M. 4th HTACG symposium, Rome, November, 2001.
- Mochida S, Hasegawa T, Tanaka R. RAN95, Int. Symposium on Advance Energy Conversion Systems and Related Technologies, Nagoya, Japan, 4-6 December 1995.
- Yuan J, Kobayashi Y. Naruse I, 2nd International High-Temperature Combustion. Symposium, Kaohsiung, Taiwan, January 20-22. 1999.
- Dong W, Blasiak W. Large eddy simulation of a single jet flow in highly preheated and dilute air combustion. Archive combustions. 2000; 20.
- Yang W, Blasiak W.. 3rd Int. Symposium on Advanced Energy Conversion Systems and Related Technologies. Dec.15-17, 2001, Nagoya, Japan.
- Tsuji H, Gupta A, Hasegawa T, Katsuki M, Kishimoto K, Morita M. Energy Conservation to Pollution Reduction. CRC Press LLC, New York, 2003.
- Mortberg M, Gupta AK, Blasiak W. Joint Conference on Sustainable Energy and Environment (SSE), December 1-3, 2004, Hua-Hin, Thailand.
- Rafidi N, Blasiak W, Jewartowski M, Szewczyk D. IFRF Electronic Combustion Journal, June 2005, article number 20050.
- Tian Y, Zhou X, Ji X, Bai J, Yuan L. Applying moderate or intense low-oxygen dilution combustion to a co-axial-jet I-shaped recuperative radiant tube for further performance enhancement. Energy. 2019; 171: 149-160.
- Roy R, Gupta AK. Flame structure and emission signature in distributed combustion. Fuel. 2020; 262: 116460.
- Karyeyen S, Feser JS, Jahoda E, Gupta AK. Development of distributed combustion index from a swirl-assisted burner. Applied Energy. 2020; 268: 114967.
- Khalil AE, Gupta AK. Towards colorless distributed combustion regime. Fuel. 2017; 195: 113–122.
- Khalil AEE, Ashwani K, Gupta AK. Fuel property effects on distributed combustion. Fuel. 2016; 171: 116–124.
- Schaffel-Mancini N, Mancini M, Szlek A, Weber R. Novel conceptual design of a supercritical pulverized coal boiler utilizing high temperature air combustion (HTAC) technology. Energy. 2010; 35: 2752-2760.
- Xing F, Kumar A, Huang Y, Chan S, Ruan C, Gu S, Fan X. Flameless combustion with liquid fuel: A review focusing on fundamentals and gas turbine application. Applied Energy. 2017; 193: 28-51.
- Khidr KI, Eldrainy YA, EL-Kassaby MM. Towards lower gas turbine emissions: Flameless distributed combustion. Renewable and Sustainable Energy Reviews. 2017; 67: 1237–1266.
- Perpignan AAV, Rao AG, Roekaerts DJEM. Flameless combustion and its potential towards gas turbines. Progress in Energy and Combustion Science. 2018; 69: 28-62.
- Stasiek J, Jewartowski M, Yang W. Small Scale Gasification of Biomass and Municipal Wastes for Heat and Electricity Production using HTAG Technology. E3S Web of Conferences. 2017; 13: 03005.
- Stasiek J, Szkodo M. Thermochemical Conversion of Biomass and Municipal Waste into Useful Energy Using Advanced HiTAG/HiTSG Technology. Energies. 2020; 13: 4218.
- Stasiek J, Baranski J, Jewartowski M, Wajs J. Gasification of densified biomass (DB) and Municipal solid wastes (MSW) using HTA/SG technology. Processes. 2021; 9: 2178.