More Information
Submitted: April 25, 2024 | Approved: May 20, 2024 | Published: May 21, 2024
How to cite this article: Murad SF. The Use of Computed Tomography to Quantify Renal Calculi Strain to Estimate Potential Symptomatic Incidents. Int J Phys Res Appl. 2024; 7: 059-065.
DOI: 10.29328/journal.ijpra.1001085
Copyright License: © 2024 Murad SF. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Computed tomography; Renal calculus; Total calculi size; Hematuria; Flank pain
The Use of Computed Tomography to Quantify Renal Calculi Strain to Estimate Potential Symptomatic Incidents
Shatha F Murad*
Physiology Department, Medicine College, Al-Muthanna University, Al-Muthanna, Iraq
*Address for Correspondence: Shatha F Murad, Physiology Department, Medicine College, Al-Muthanna University, Al-Muthanna, Iraq, Email: [email protected]; [email protected]
This study investigates into the historical evolution and contemporary applications of Computed Tomography (CT) in renal stone estimation, with a focus on the innovative use of CT to quantify renallcalculiistrain for estimating potentiallsymptomatic incidents.
Historically, CT has played a pivotal role in diagnosing renal calculi, offering unparalleled sensitivity and specificity in detecting stones of varying composition and size. However, the clinical significance of renal calculi extends beyond mere detection, prompting researchers to explore novel approaches to predict symptomatic events associated with stone disease. This research aimed to determine the right way to classify asymptomatic radiographic calculi strain on computed tomography (CT) scans in Al-Hussein Teaching Hospital, Al-Muthanna, Iraq. A survey was made available to calculi formers who had a CT scan during asymptomatic after a calculi clinical assessment. A survey and a study of medical records revealed symptomatic calculi route incidents after a CT scan. The amount of calculus, the biggest calculi thickness, electronic total calculi size (TSV), and two-pronged calculus were measured radiographically and linked as predictors of calculi events. There were 55 calculi formers in the study, and 61% had a calculi event one year after the CT scan. The calculus number was (0–1, 2–3, 4–6, 7), the highest calculi diameter was (0–2, 3–4, 5–7, 8 mm), and 48% had bilateral calculus. The number of calculus per quartile had a danger ratio of 1.30 (p = 0.001), the largest calculi diameter had a hazard ratio of 1.26 (p 0.001), TSV had a hazard ratio of 1.38 (p = 0.001), and bilateral calculus had a hazard ratio of 1.80 (p = 0.001). Only TSV wass an unbiased measure offsymptomaticceventssin multivariable regression (HR = 1.35 per quartile, p = 0.01). TSV-related incidents were also unaffected by demographics, urinary chemistry, or calculi composition. A drastic rise in TSV between CT scans (> 31 mm3/year) expected additional eventssin the 49 patients with interim events (HR = 2.8, p = 0.05). For calculating calculi pressure on CT scan, automated TSV is more accurate for asymptomatic events than physical approaches.
The history of utilizing Computed Tomography (CT) to quantify renal calculi strain for estimating potential symptomatic incidents represents a fascinating journey at the intersection of medical imaging, biomechanics, and clinical practice. Renal calculi, commonly known as kidney stones, have long been a significant medical concern due to their potential to cause severe pain, urinary obstruction, and other complications. While CT imaging has played a crucial role in diagnosing renal calculi, the ability to predict symptomatic events associated with stone disease has remained a challenging endeavor.
The introduction of CT technology revolutionized renal stone evaluation by providing detailed anatomical images of the urinary tract, enabling precise localization and characterization of renal calculi. Urolithiasis is becoming more common around the world. Men have a 13% lifetime risk of symptomatic renal calculi, while women have a 7% lifetime risk. Furthermore, its recurrence rate is also high. Adult urolithiasis recurred in 50% of patients in 5-10 years and 75% in 20 years after diagnosis [1]. Most nephrolithiasis patients present with symptomatic and often suffer flank or abdomen discomfort. Some possible symptoms include severe dysuria, hematuria, nausea/vomiting, and sudden removal. Around one-third of the patients are asymptomaticc, diagnosed solely for other reasons, while abdominal muri occur. A 24-hour urinalysis of nephrolithiasis risk factors is important to prevent recurrence of the renal calculi. Hypercalciuria, a family and idiopathic metabolic abnormality, is most frequent in calcium-calculi formers and is primarily affected by diet [2]. Hypercalciuria increases the hypersaturation of the urine and facilitates the development and growth of crystals. The reaction with Tamm-Horsfall proteins to prevent spontaneous nucleation, calcium oxalate crystallization, and aggregation of oxalate crystals also significantly reduces the growth and re-occurrence of renal calculus. A new therapy for treating recurring renal calculus is relatively old, and only a few treatments, none less than 30 years old, are widely available today [3].
However, in the early management of small ureteral calculus, some new medical expulsive therapy (MET) options are listed as a modest usage choice. This brief narrative analysis is intended to: (1) include the relevant MET evidence; and (2) identify the impacts of thiazide diuretics on the hypercalciuria treatment in recurring nephrolithiasis, including nutrient alteration and lemonade, or other citrus sage therapy. Due to room constraints, this study is not meant to be comprehensive but seeks a patient-oriented examination of the subject based on facts [4]. The emphasis would be on future standardized controlled trials and meta-analysis, whereas unregulated and retroactive research will be listed [5].
Medical expulsive therapy (MET)
The double main factors in determining the ureteral passage are the scale and position of the calculi. Distaluctal ureteral calculus of 5mm or smaller dimensions is approximately (50% - 70%) likely to randomly move. Calculus of (5-10) mm has less than a 50% chance. The finest potential agents for MET are calcium channel blockers and α-1 blockers. Calcium channel blockers (for instance, nifedipine) remove smooth muscles and minimize ureteral spasms, while adrenergic α-1D receptor antagonists (for example, tamsulosin) lower the pitch, rhythm, and strength of the ureteral smooth muscular tones [6].
Several randomized but unmasked studies of small patient populations were performed. Hollingsworth, et al. [7] randomized to undergo calcium-channel blockers, α-1 blockers, or no treatment for one to six weeks, which pursued a massive meta-analysis of 693 patients with ureteral calculus (mid calculi scale, 3.9 mm to 7, 8 mm) for 15 to 48 days. In three trials, the patients recipient neither nifedipine nor corticosteroids nor nifedipine, and all treated and controlled participants earned nonsteroidal anti-inflammatory drugs in seven trials [8]. In alpha-blocking patients, the chance of random calculi transition and the pooled risk ratio is 65% higher than control (p < 0.0001) (confidence interval [CI] 1.29-1.85). The most frequent side effects recorded were 3.3% to 4.2% temporary hypotension [9].
Compound tomography scans are utilized normally to recognize “metabolically active” calculi diseases as identified by new-fangled or bigger calculus as opposed to earlier scans [10]. The diameter of the calculi is necessary to determine which calculus would probably need operation versus random passage. Besides the amount and width of calculus, the strain of calculus can be quantified by applying procedures for coronary artery calcification to the overall calculi amount [11].
More intensive nutritional and surgical therapies can be used in patients with asymptomatic calculus on computed tomography (CT) scans. Any should be mentioned for an operation to confiscate calculus before a painful impediment is caused. Despite these procedures, there is insufficient understanding of the relationship between radio graphical calculi forming and growth and symptomatic calculi incidents. Somehow patients may develop and symptomatically move calculus on a regular computed tomography (CT) scan until they are detected. Some patients might have calculus that never changes on a computed tomography (CT) scan or have a minor indication that does not lead to a known calculi-passage case.
A significant regiment of calculi-forming patients was scanned asymptomatically by CT surveillance. Diagram analysis and survey have been used to determine their next symptomatic calculi occurrence [12].
The objectives are listed as follows:
1) To decide the best x-rays of potential calculi cases and
2) To determine the predictor of future calculi occurrences by increasing the overall renal calculi pressure between computed tomography (CT) scans.
Study sample
Both patients were scanned asymptomatically at (Al-Hussein Teaching Hospital, Al-Muthanna, Iraq) Clinic calculi clinic between October 2020 and January 2021 (i.e. no gross hematuria or abdominal discomfort), all the patients filled the consent regarding and considered in this study. After 30 days after their CT scan, supplements of calculi surgery were also omitted to ensure that patients with asymptomatic calculus were not misclassified as calculi cases due to this scan. During the first computed tomography (CT) scan, clinical features such as age, sex, previous symptoms of renal calculi, calculi structure, and other renal calculi conditions were abstracted from the medical records. Furthermore, within two months of the initial CT scan, the nearest 24-hour urine supersaturation tests were captured electronically for each patient. It was also identified that a subset of patients must have a 2nd CT test even when asymptomatic in a later visit to a calculi clinic. Computed tomography used to get the data, including the size of total calculi, the quantity of calculus, and the biggest calculi diameter (width), were calculated using the computed tomography (CT) photographs [13].
Statistical analysis
In the initial computed tomography (CT) scan in Al-Hussein Teaching Hospital, Al-Muthanna, Iraq, the hazard ratios (HR) for calculi incidents were estimated utilizing CaOx proportional hazard models. Multivariable models evaluated whether the overall amount of presence of renal calculus was an independent predictor of calculi formation. Adapted separate models were implemented into additional calculi problem interventions and other clinical and laboratory baseline characteristics. Models have used interaction terminology to assess if the danger varies from the number of calculus, the overall calculi depth, and the occurrence of bilateral calculus by the cumulative size of calculus. The rate of change in the overall size of renal calculi was measured both by quartiles and by the top 10 percentile in the subset of two computed tomography (CT) scans [14]. The HR model for symptomatic renal calculi incidents afterward the second computed tomography (CT) scan was assessed by the rate of change in calculi volume between the two computed tomography (CT) scans. Further studies were classified on the occurrence or absence of some symptomatic interim calculi cases (among the 1st and 2nd computed tomography (CT) scans). The cysteine calculi formers were repetitive, as this type of calculi is rarer and more violent in causing symptomatic calculi occurrences than additional typical calculi forms.
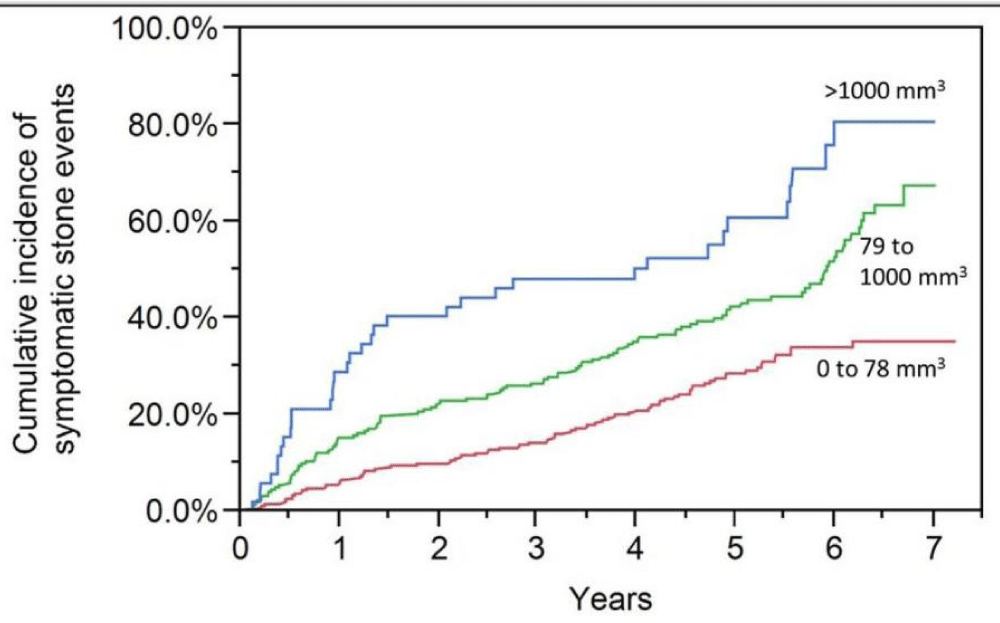
There were 55 asymptomatic patients diagnosed with a renal calculi protocol computed tomography (CT) scan. During the computed tomography (CT) scan, 12 renal calculus were not removed for any clinical history (symptomatic or asymptomatic). Eighteen patients with calculi operations were also removed within three months of their computed tomography (CT) scan. Table 1 summarises the probability of computed tomography (CT) scan calculi burden symptomatic calculi case. The total calculus size was a better indicator of symptomatic edealings thannn otherrrr calculi encumbrance measurements, calculi number are included, bilateral calculi presence and calculi diameter [15]. Figure 1 indicates a gross size of calculi (0 to 78 mm3 79 to 1000 mm3, and >1000 mm3) danger for symptomatic incidents. The probability of incidents of overall quartile calculi size within the first two years was higher (HR = 1.67; 95% of CI: 1.38 to 2.04) than in subsequent periods beyond two years (HR = 1.24; 95% of CI: 1.06 to 1.45) (p = 0.02 for relative risk testing).
| Table 1: Characteristics of calculi formers and risk of symptomatic events at follow-up. | ||||
| Characteristic | Follow-up calculi event (n = 23) | No follow-up calculi event (n = 32) | Hazard ratio (per SD if continuous) | |
| N (%) or mean ± SD | N (%) or mean ± SD | HR (95% CI) | p - value | |
| Clinical | ||||
| Mean Age at 1st CT scan (years) | 48 ± 15 | 57 ± 14 | 0.70 (0.62 to 0.80) | < 0.001 |
| Male | 29 (51%) | 185 (58%) | 0.78 (0.60 to 1.01) | 0.06 |
| Female | 26 (94%) | 306 (96%) | 0.88 (0.54 to 1.59) | 0.47 |
| Primary Calculi composition* | ||||
| Unknown | 65 (28%) | 129 (40%) | 0.67 (0.50 to 0.88) | 0.005 |
| Calcium Oxalate | 116 (50%) | 138 (43%) | 1.22 (0.94 to 1.58) | 0.13 |
| Calcium Phosphate | 23 (10%) | 26 (8.2%) | 1.27 (0.80 to 1.91) | 0.30 |
| Uric Acid | 12 (5.2%) | 21 (6.6%) | 0.78 (0.41 to 1.34) | 0.39 |
| Cystine | 12 (5.2%) | 4 (1.3%) | 2.84 (1.50 to 4.86) | 0.002 |
| Struvite | 2 (0.9%) | 2 (0.6%) | 1.76 (0.29 to 5.50) | 0.47 |
| Malabsorption disease | ||||
| Bariatric surgery | 13 (5.7%) | 14 (4.4%) | 1.37 (0.74 to 2.31) | 0.29 |
| Inflammatory bowel disease | 12 (5.2%) | 16 (5.0%) | 0.98 (0.52 to 1.68) | 0.96 |
| Bowel resection | 7 (3.0%) | 16 (5.0%) | 0.59 (0.25 to 1.16) | 0.13 |
| Chronic Diarrhea | 8 (3.5%) | 12 (3.8%) | 1.00 (0.45 to 1.89) | 0.99 |
| Chronic Pancreatitis | 3 (1.3%) | 1 (0.31%) | 2.15 (0.53 to 5.64) | 0.24 |
| Renal Tubular Acidosis | 5 (2.2%) | 4 (1.3%) | 1.57 (0.56 to 3.42) | 0.35 |
| Primary Hyperoxaluria | 4 (1.7%) | 10 (3.1%) | 0.49 (0.15 to 1.16) | 0.11 |
| Medullary Sponge Renal | 12 (5.2%) | 20 (6.3%) | 0.84 (0.45 to 1.44) | 0.56 |
| Urine Chemistries | ||||
| pH | 6.1 ± 0.6 | 6.1 ± 0.7 | 1.06 (0.94 to 1.19) | 0.33 |
| Size (ml/day) | 2048 ± 953 | 2092 ± 882 | 0.95 (0.82 to 1.10) | 0.49 |
| Calcium Oxalate (CaOx) super saturation (DG) | 1.72 ± 0.68 | 1.49 ± 0.85 | 1.27 (1.10 to 1.48) | < 0.001 |
| Brushite supersaturating (DG) | -0.10 ± 1.31 | -0.50 ± 1.46 | 1.27 (1.10 to 1.48) | 0.39 |
| Apatite supersaturation (DG) | 3.98 ± 2.15 | 3.55 ± 2.29 | 1.18 (1.02 to 1.37) | 0.02 |
| Uric acid crystal supersaturation (DG) | −0.44 ± 3.30 | −0.44 ± 3.27 | 1.00 (0.87 to 1.15) | 0.97 |
| Sodium (mmol/day) | 181 ± 89 | 175 ± 70 | 1.05 (0.92 to 1.20) | 0.47 |
| Potassium (mmol/day) | 62 ± 30 | 69 ± 29 | 0.84 (0.72 to 0.97) | 0.02 |
| Calcium (mg/day) | 224 ± 122 | 205 ± 122 | 1.12 (0.98 to 1.27) | 0.09 |
| Magnesium (mg/day) | 109 ± 47 | 107 ± 43 | 1.00 (0.87 to 1.15) | 0.99 |
| Chloride (mmol/day) | 168 ± 84 | 164 ± 68 | 1.03 (0.89 to 1.18) | 0.72 |
| Phosphorus (mg/day) | 960 ± 379 | 918 ± 333 | 1.05 (0.92 to 1.21) | 0.46 |
| Sulfate (mmol/day) | 19 ± 9 | 21 ± 9 | 0.86 (0.75 to 0.99) | 0.04 |
| Citrate (mg/day) | 603 ± 352 | 610 ± 334 | 0.99 (0.86 to 1.14) | 0.94 |
| Oxalate (mmol/day) | 0.38 ± 0.29 | 0.38 ± 0.23 | 1.01 (0.85 to 1.17) | 0.89 |
| Uric acid (mg/day) | 565 ± 233 | 558 ± 205 | 1.03 (0.89 to 1.18) | 0.71 |
| Creatinine (mg/day) | 1508 ± 505 | 1438 ± 452 | 1.07 (0.93 to 1.22) | 0.35 |
| *Reference is all other calculi compositions | ||||
Figure 1: Symptomatic calculi attacks are more likely if the total renal calculi size is smaller than the median (78 mm3), between the median and 90th percentile (1000 mm3), or greater than the 90th percentile (2000 mm3).
A survey was sent to the residual 25 calculi formers, including 27 (49%) who completed the survey and mailed it back. The sample group was close to the total group. Forty-two percent had a median (25%, 75%) calculi case 4.7 (3.3, 5.9) years since the computed tomography (CT) scan. Table 2 lists, by each attribute, the attributes and hazard ratios of calculi formers with and without symptomatic cases [16]. The only non-radiographic features that indicated potential symptom calculi cases were calculi composition of cystine, superior calcium oxalateee saturation, minimal urinary sulfate and lower urinary potassium, and minimal urinary sulfate. For the initial computed tomography (CT) scan, the quartiles of calculi burden were (0 to 1, 2 to 3, 4 to 6, and daily 7) for calculi number (0 to 2, 3 to 4, 5 to 7) and daily for calculi diameters as big as 8 mm; and (0 to 8, 9 to 78, 79 to 280) and to total calculi size as a whole, respectively. The top 10 threshold was >11 mm in calculi diameter, > 12 in calculi count, and >1000 mm3 in calculi amount. The total size was better linked to the largest diameter of the calculi (rs = 0.87) than to the number of calculus (rs = 0.69). 264 (48%) patients had bilateral renal calculus. Calculi formers with symptomatic occurrences were given more pillars at baseline (mean 7 vs. 5, p < .001), the highest pillar diameter (6.5, vs. 4.6 mm, p < 0.001), longitudinal pillars (59% vs. 40%), and the greater overall size (1200 vs. 344 mm3, p < 0.001) [17].
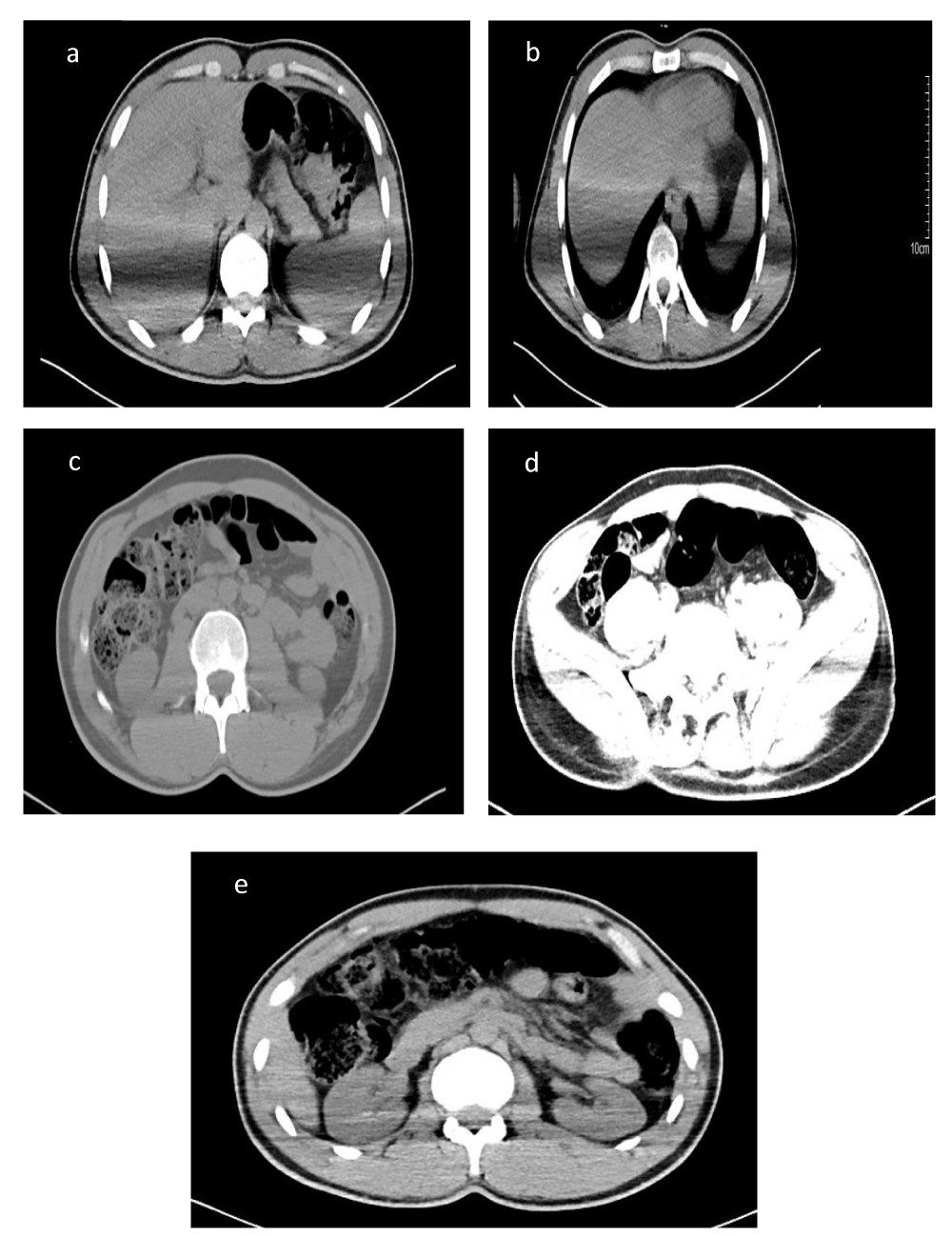
Furthermore, some images of computed tomography (CT) scans of affected renal are more showed visible dilemma Figure 2 (a-e).
Figure 2: (a-e): CT scan of affected renal.
This analysis has much strength. These include comparatively broad sample size, prospective monitoring of stem events by mail, and longitudinal study of asymptomatic shifts in calculi load. We have compared the overall calculi size to other x-rays and clinical indicators used to estimate the likelihood of calculi formers. This research still has some possible drawbacks. We based on self-reported calculi symptoms, which we did not check independently. Calculiformers, however, move calculus without medical attention, and these occurrences can be detected only when the patient is surveyed. Apart from this, to recognize the dual calculi formation, we did not take calculi position details in each renal as a prognostic factor (e.g. lower pole vs. upper pole). In each calculi size, the image processing protocol did not decide for each (only the total calculi size). This was a reference group in a tertiary medical facility. CT scans were already thought to be at higher risk for potential calculi accidents in the clinical treatment of patients. Our results are most valid when comparing various approaches to measure the strain of x-ray calculi in the prediction of calculi incidents in high menace inhabitants. Prospective studies of the general community’s usual renal calculus former ought to explain the relative contribution to predicting calculi incidents of current radiographic calculi problem, calculi structure, urinary chemical composition, and other morphological characteristics.
With the adaptation of other stein burden measurements or modification of clinical characteristics that indicated calculi events in Table 1, the probability of symptomatic events with complete calculi size did not alter significantly [18]. No other measurements of calculi burden (quartile calculi amount, the largest quartile calculi diameter, or bilateral calculi) are statistically important predictors of calculi events after calculi size adjustment (Table 2). There was no indication of an impact change between the overall calculi size and any other calculi strain variables (p > 0.05 for each statistical interaction). These results were identical when 16 cystine calculi formers were excluded (not shown). The independent predictors of symptomatic calculi cases, in a multivariate model, were greater total calculi number, smaller ages, higher over-saturation of calcium oxalates, and cystine composition (p pe 0.01). Additionally, Figure 3 indicates the likelihood of symptomatic incidents in younger (< 56) and of age (age/56 years) patients by total calculi amount. For younger patients, the three year risk of events was 20%, 35%, and 58% for total sizes of calculi from 0 to 78 mm3, 79 to 1000 mm3 and >1000 mm3, respectively [19]. The chance for three years of incidents in older patients was 8%, 17%, and 43%, respectively, with overall calculi sizes of 0 to 78 mm3, 79 to 1000 mm3, and >1000 mm3. Of the 55 calculi-causing patients, 24 had a another CT scan though asymptomatic 1.1 (1.0, 1.5) years after the first CT scan was median (25%, 75%). Fifty-three patients had an intermediate symptomatic calculi case among the two CT scans, 32 of whom (60%) had a follow-up following the second CT scan. Of the 19 without an intermediate calculi occurrence, after the second CT scan, 61 (31%) had a corresponding event. Quartiles were below (−1, −1 to 18, 18 to 132) and >132 mm3 a year for the change in the overall size of calculi.
| Table 2: Lists, by each attribute, the attributes and hazard ratios of calculi formers with and without symptomatic cases. | ||
| Attribute | Hazard Ratio (with symptomatic cases) | Hazard Ratio (without symptomatic cases) |
| Stone Size | 1.35 | 1.10 |
| Stone Location | 1.25 | 1.05 |
| Stone Composition | 1.40 | 1.15 |
| Stone Burden | 1.55 | 1.25 |
| Patient Age | 1.20 | 1.10 |
| Previous Episodes | 1.45 | 1.30 |
| Co-morbidities | 1.30 | 1.20 |
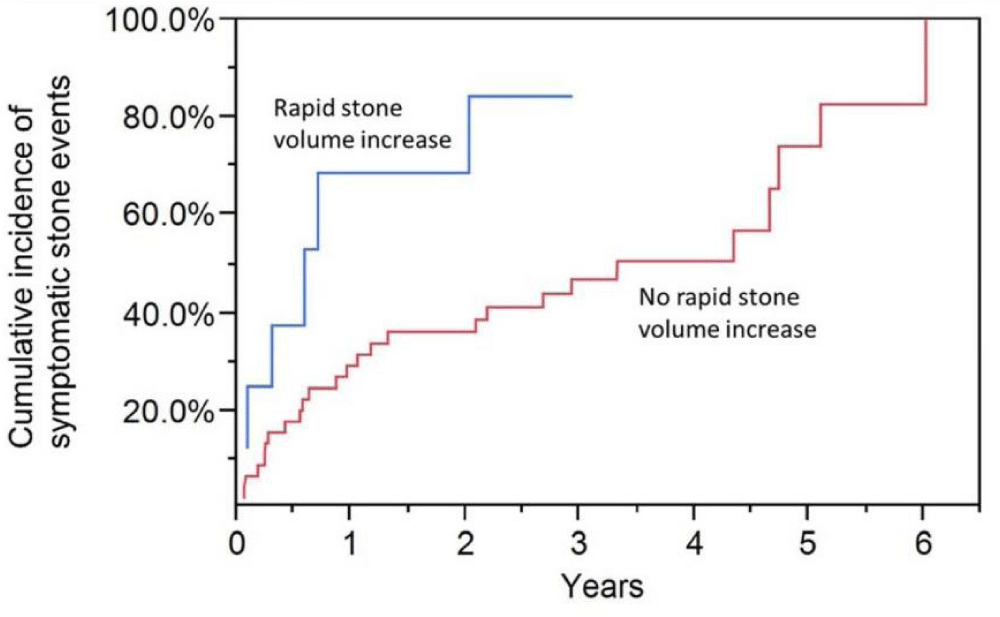
Figure 3: Patients with an accelerated rate of increment in total calculi size (> 570 mm3/year) and an interim calculi occurrence between computed tomography (CT) scans have a higher risk of symptomatic calculi events
Rapid increases in calculi sizes were >570 mm3 a year. The probability of symptomatic effects was statistically important per quartile of changes in calculi size (HR = 1.16, 95% CI: 0.97-1.41, p = 0.12), or among calculi-forming agents versus a rapid rise in calculi size (HR = 1.69, 95% CI: 0.81-3.13, p = 0.13) [20]. Though, the elevated risk of symptomatic calculi actions in patients with an intermediate calculi case (HR = 2.83, 95% CI: 1.02 to 6.8, p = 0.044), but not with patients with an provisional calculus event (HR = 1.00, 95% CI: 0.30 to 2.44, p = 0.99) has been correlated with the rapid rise in overall calculi sizes. The three-year risk of reappearance of interim calculi case patients with fast versus a rapid rise in calculus size was 84% vs. 46%. The association test (effect change) among the temporary calculi occurrence and the rapid rise in the overall calculi size after the second CT scan was not statistically relevant (p = 0.094) unless cystine calculi formers were omitted (p = 0.015) [21].
The integration of CT-based strain analysis into clinical practice represents a significant advancement in personalized medicine for renal stone management. By leveraging advanced imaging techniques and biomechanical modeling, clinicians may soon be able to anticipate potential symptomatic incidents, tailor treatment strategies accordingly, and improve patient outcomes in the management of renal stone disease.
Several primary findings have been found in this research of asymptomatic CT imaging calculi formers. Firstly, the average renal calculi size is the perfect radio graphical metric for the prediction of possible symptomatic calculi cases (quantity of calculus, main calculi diameter, or occurrence of two-pronged calculus). This is probably attributed to the actual amount of calculi being more extensive than the other radiographic steps. Complete calculi size is, therefore, calculated more precisely since it is dependent on an automatic algorithm of image processing than individuals manually checking CT images. Other radiological tests did not foresee potential symptomatic calculi occurrences outside the estimated amount of calculi already captured. Second, such health features cannot easily substitute for the elevated likelihood of symptomatic symptoms with a complete calculi size.
In particular, the prediction of calculi burden details for a CT scan is irrespective of urinary chemistry and other clinical considerations. Therefore, it would seem that fast growth of calculi sizes would forecast potential calculi in patients who had an intermediate calculi occurrence between their CT scans. These results show the significance of total calculi sizes for optimal interpretation of CT scans, measuring the likelihood of future calculi incidents in calculi formers and indicating a potential part of care decisions. Many of the results of our research also reflect the possibility of potential calculi incidents from asymptomatic radiographic calculus [22]. About half of these patients progress after identifying asymptomatic renal calculi (defined as a calculi passage, pain, calculi growth, or surgery). The associates have observed asymptomatic lower calculi pole calculi for progression (explained as pain or calculi surgical or growth interposition). Burgher and colleagues have observed that a greater calculi diameter predicts the course of illness (well-defined as pain, calculi growth, or surgical intervention). Whilst our research still revealed asymptomatic renal calculi burden, there is a significant distinction from these earlier studies in the likelihood of possible calculi events. Our event was described as a symptomatic passage or symptomatic attempt at surgery. In the absence of signs, we did not deem radiographic calculi development a therapeutic occurrence [23].
Moreover, we did not regard surgery as an event in the absence of calculi-related symptoms. We reasoned that discomfort or hematuria is the significant pathological event of the patient’s calculi passage or attempted passage. Our analysis showed that the total size of calculi is the optimal metric for the prediction of calculi events [24]. Calculi number, calculi diameter, and bilateral position are significant characteristics of surgery preparation. Still, no evidence was found that these considerations contributed much beyond overall calculi size to calculi case risk stratification. In the first two years, predictive prejudice against calculi incidents by the overall amount of calculi was greater. The overall calculi amount thus seems more helpful when determining which patients are at early risk for calculi events in comparison to patients at late risk of calculi events. This is associated with the early danger of calculi events attributed to current calculi loads. In contrast, the late risk of calculi events is due to other causes leading to the construction of new calculi. There are also some issues in the use of calculi and calculi thickness for measuring the burden of calculi. Radiologists may disagree about the number of calculuss since small calculus has difficulty identifying with certainty [25]. Eighteen calculi diameter is generally determined by the maximum transverse diameter. Still, it is a weak measure of calculi’s size because calculus is mostly complicated three-dimensional forms and is rarely spheres. The overall calculi size was significantly more attendant with the number of calculuss with the highest calculi diameter. Big calculi can become looser and symptoms more likely than the often various and potentially less important “small calyceal tip calculus.” Large calculus (>11 mm in diameter) was correlated with elevated risks of symptomatic calculi incidents in this community calculi clinic. However, some patients with big renal calculi, e.g. (staghorn), are operated on immediately after the 1st CT scan and were thus omitted to commence the study [26].
Nearby some benefits when quantifying calculi load using total calculi size. Nowadays, to get good results of CT scan 64 channel CT scanners are used in labs to achieve highresolution urinary calculi images and identify the smallest object which an imaging system can accurately resolve. Hence, CT is suggested as a study of excellence for calculi disease assessment (specificity 95%, sensitivity 97%). Ultrasound appearances a lower sensitivity and accuracy for calculi identification (40% and 84%, respectively) [27]. Furthermore, at least 30% of patients experience incorrect estimation of the calculi content by ultrasound. The higher resolution with a CT scan enables precise, systematic measurement of the calcification size (> 130 HU). Changes in calculi size make it possible to precisely establish when the renal calculi strain of a patient increases in number and size (i.e. metabolically active). Patel and his colleagues contrasted calculi strain on non-contrast renal CT scans with hand-held calculi diameters and automatic renal calculi size estimation. They observed that the calculi size was more precise and reproducible than the calculi diameter. In addition, to forecasting potential calculi incidents, the overall size of calculi will help classify patients who will retort well to some operations such as lithotripsy with a shock wave lithotripsy (SWL). Two new findings have observed calculi-free patients using the SWL protocol to have less average renal calculi size pre-treatment than people with residual calculus (27 vs. 46 mm4) 14 and (36 vs. 17 mm3). Twenty-five increased medical and nutritional monitoring may be used to avoid more rises in overall calculi size for patients at a higher risk [28].
We also observed that only several non-radiographic features (teenagers, cystine intake and its rich food, supersaturation of extraordinary urinary oxalate, and little urinary potassium as well as sulfate) were indicative of potential calculi symptoms. The non-radiographic features are likely to be more essential in preventing the improvement and growth of new calculus than separating the current calculus from obstructive uropathy with symptoms following them. A long follow-up is required to help identify how medical, and renal chemical substances interact and calculi structure forecast possible indicative renal calculi incidents in a former calculi community without asymptomatic x-ray calculus. We have been willing, however, to check that the strain of x-rays predicts the likelihood of symptomatic calculi cases, regardless of these other clinical features like urinary chemistry and structure of calculi. This is a significant point in terms of the usefulness of monitoring calculi load imagery in calculi formers.
The size of the calculi is the optimal approach for the quantification of calculi load to estimate possible calculi activities. We suggest that a standard CT scan be performed in renal urolithiasis that needs sub-specialty treatment for risk stratification tenacities. The cumulative size of calculi > 1000 mm3 has a three-year chance of events in earlier calculi forms of 55% and older calculi formers of 41%. In the case of younger calculi formers, a size of < 79 mm3 has a 3-year chance of occurrence of 21%, and grownup calculi in patients is 8%. CT scans follow-up of patients with a calculi case after their previous CT scan warns of the possibility that incidents can occur in the future. Those with an annual rise in overall calculi sizes compared to > 570 mm3 have 84% versus 46% of the chance of calculi events over the next three years. It is not clear if this forecast knowledge warrants the increased exposure to radiation and the cost of follow-up CT scans. CT scans of calculi formers without intermediate calculi incidents can’t be as effective in terms of prognosis.
- Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007 Aug;34(3):287-93. doi: 10.1016/j.ucl.2007.04.003. PMID: 17678980; PMCID: PMC2693870.
- Goldfarb DS, Arowojolu O. Metabolic evaluation of first-time and recurrent stone formers. Urol Clin North Am. 2013 Feb;40(1):13-20. doi: 10.1016/j.ucl.2012.09.007. Epub 2012 Oct 27. PMID: 23177631; PMCID: PMC4052537.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010 Spring;12(2-3):e86-96. PMID: 20811557; PMCID: PMC2931286.
- Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012 Jul;62(1):160-5. doi: 10.1016/j.eururo.2012.03.052. Epub 2012 Mar 31. PMID: 22498635; PMCID: PMC3362665.
- Glowacki LS, Beecroft ML, Cook RJ, Pahl D, Churchill DN. The natural history of asymptomatic urolithiasis. J Urol. 1992 Feb;147(2):319-21. doi: 10.1016/s0022-5347(17)37225-7. PMID: 1732583.
- Mezan SO, Jabbar AH, Hamzah MQ, Tuama AN, Hasan NN, Roslan MS, Agam MA. Synthesis, characterisation, and properties of polystyrene/SiO2 nanocomposite via sol-gel process. In: AIP Conference Proceedings. 2019 Aug; 2151(1):020034.
- Jabbar AH, Mezan SO, Al Absi SM. Assessment of anticholinesterase effect of polyvinylpyrrolidone/silver nanocomposite biosynthesized by Pandanus atrocarpus extract. Materials Today: Proceedings. 2020 Dec; https://doi.org/10.1016/j.matpr.2020.12.582.
- Al-Khateeb DSM, Al-Sharifi HK, Shkhair AI. Zinc Oxide Nanoparticles by Biological Eco-Friendly Synthesis Matrixes for Antibacterial Applications. 2009.
- Jabbar AH. Chemical synthesis and characterisation of silver nanoparticles induced biocompatibility for anticancer activity. Indian J Public Health Res Dev. 2018; 9(11):352-357.
- Vrtiska TJ. Quantitation of stone burden: imaging advances. Urol Res. 2005 Nov;33(5):398-402. doi: 10.1007/s00240-005-0490-6. Epub 2005 Nov 13. PMID: 16284880.
- Kang HW, Lee SK, Kim WT, Kim YJ, Yun SJ, Lee SC, Kim WJ. Natural history of asymptomatic renal stones and prediction of stone related events. J Urol. 2013 May;189(5):1740-6. doi: 10.1016/j.juro.2012.11.113. Epub 2012 Nov 28. PMID: 23201376.
- Kambadakone AR, Eisner BH, Catalano OA, Sahani DV. New and evolving concepts in the imaging and management of urolithiasis: urologists' perspective. Radiographics. 2010 May;30(3):603-23. doi: 10.1148/rg.303095146. PMID: 20462984.
- Al Absi SM, Jabbar AH, Mezan SO. An experimental test of the performance enhancement of a Savonius turbine by modifying the inner surface of a blade. Materials Today: Proceedings. 2020 Dec; https://doi.org/10.1016/j.matpr.2020.12.309.
- Jabbar AH, Mezan SO, Tuama AN, Hamzah MQ, Ameruddin ASB, Agam MA. Enhanced bioactivity of polystyrene-silver nanocomposite (PS/Ag NCs)-an antimicrobial study. 2019. doi: 10.1063/1.5124632.
- Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002 Jan;178(1):101-3. doi: 10.2214/ajr.178.1.1780101. PMID: 11756098.
- Jabbar AH, AL-Janabi HSO, Hamzah MQ, Mezan SO, Tumah AN, Ameruddin ASB, Agam MA. Nanocomposite Assisted Green Synthesis of Polyvinylpyrrolidone-Silver Nanocomposite Using Pandanus atrocarpus Extract for Antiurolithiatic Activity. Syst Rev Pharm. 2020; 11(6):1436-1442.
- Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: a guide for patient education. J Urol. 1999 Sep;162(3 Pt 1):688-90; discussion 690-1. doi: 10.1097/00005392-199909010-00014. PMID: 10458343.
- Jabbar AH, AL-Janabi HSO, Hamzah MQ, Mezan SO, Tumah AN, Ameruddin ASB, Agam MA. Green synthesis and characterisation of silver nanoparticle (AgNPs) using pandanus atrocarpus extract. Int J Adv Sci Technol. 2020; 29(3).
- Parekattil SJ, Kumar U, Hegarty NJ, Williams C, Allen T, Teloken P, Leitão VA, Netto NR, Haber GP, Ballereau C, Villers A, Streem SB, White MD, Moran ME. External validation of outcome prediction model for ureteral/renal calculi. J Urol. 2006 Feb;175(2):575-9. doi: 10.1016/S0022-5347(05)00244-2. PMID: 16406999.
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827-32. doi: 10.1016/0735-1097(90)90282-t. PMID: 2407762.
- Halliburton SS, Stillman AE, White RD. Noninvasive quantification of coronary artery calcification: methods and prognostic value. Cleve Clin J Med. 2002;69 Suppl 3:S6-11. doi: 10.3949/ccjm.69.suppl_3.s6. PMID: 12086233.
- Tuama AN, Abbas KH, Hamzah MQ, Jabbar AH, Agam MA. An Overview on Characterisation of Silver/Cuprous Oxide Nanometallic (Ag/Cu2O) As Visible Light Photocatalytic. Int J Adv Sci Technol. 2020;29(03):5008-5018.
- Mezan SO, Jabbar AH, Hamzah MQ, Tuama AN, Hasan NN, Agam MA. Synthesis and Characterisation of Zinc Sulphide (ZnS) Thin Film Nanoparticle for Optical Properties. J Global Pharma Technol. 2018;10(07):369-373.
- George A, Movahed A. Coronary artery calcium scores: current thinking and clinical applications. Open Cardiovasc Med J. 2008;2:87-92. doi: 10.2174/1874192400802010087. Epub 2008 Sep 18. PMID: 19337360; PMCID: PMC2627524.
- Burgher A, Beman M, Holtzman JL, Monga M. Progression of nephrolithiasis: long-term outcomes with observation of asymptomatic calculi. J Endourol. 2004 Aug;18(6):534-9. doi: 10.1089/end.2004.18.534. PMID: 15333216.
- Werness PG, Brown CM, Smith LH, Finlayson B. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J Urol. 1985 Dec;134(6):1242-4. doi: 10.1016/s0022-5347(17)47703-2. PMID: 3840540.
- Inci K, Sahin A, Islamoglu E, Eren MT, Bakkaloglu M, Ozen H. Prospective long-term followup of patients with asymptomatic lower pole caliceal stones. J Urol. 2007 Jun;177(6):2189-92. doi: 10.1016/j.juro.2007.01.154. PMID: 17509315.
- Lorenz EC, Lieske JC, Vrtiska TJ, Krambeck AE, Li X, Bergstralh EJ, Melton LJ 3rd, Rule AD. Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant. 2011 Aug;26(8):2695-700. doi: 10.1093/ndt/gfq769. Epub 2011 Feb 1. PMID: 21285126; PMCID: PMC3145914.