More Information
Submitted: 08 July 2019 | Approved: 10 July 2019 | Published: 12 July 2019
How to cite this article: Kordas G. Nanotechnology to improve the biofouling and corrosion performance of marine paints: from lab experiments to real tests in sea. Int J Phys Res Appl. 2019; 2: 033-037.
doi: 10.29328/journal.ijpra.1001012
Copyright License: © 2019 Kordas G. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Nanotechnology to improve the biofouling and corrosion performance of marine paints: from lab experiments to real tests in sea
George Kordas*
Institute of Nanoscience and Nanotechnology, NCSR Demokritos, 15310 Agia Paraskevi Attikis, Greece
*Address for Correspondence: George Kordas, Sol-Gel Laboratory, Institute of Nanoscience and Nanotechnology, NCSR Demokritos, 15310 Agia Paraskevi Attikis, Greece, Tel: +30 210 650 3301; Email: [email protected]
Nanocontainers of the type CuO, ZnO and CeMo were developed in the present work and incorporated into commercial paints. The nanocontainers were filled with bromosphaerol (CuO and ZnO), SeaNineTM 211 (CuO and ZnO), and 8Hydroxyquinoline (CeMo). The new resulting paints were tested in the lab and in the sea via painting a fraction of two ships. The outcome of this work is encouraging demonstrating that the new nanotechnology-based paints yield to superior commercial paints that may present a major milestone in the new generation of marine paints.
Biofouling is the most serious problem that maritime industries faces today. Its solution has economic, environmental and financial implications. Biofouling is the result of submerging a ship into sea water where a biofilm is formed composed of microorganisms. They grow quickly forming a surface colonized by algae and invertebrates finishing to its corrosion [1]. This results in erosion, decrease of speed, bigger fuel consumption and augmented air pollution. The International Maritime Organization (IMO) states that the world transaction fleet will be burning about half a billion tons of fuel per year by 2020. The estimates tell [2], a ship heavily fouled consumes more than 70% petrol in comparison to a ship with clean hulls. In other words, a ship efficiently protected against fouling will save more than 150 billion USD per year in 2020. A small increase of biofouling at 5% causes a 14% upsurge in emission of CO2, NOx and SOx gases [3]. It is estimated that the repair costs the maritime industries amounts to more than 6.5 billion USD per year. Today, the antifouling paints are created on copper compounds and booster biocides. They are toxic compounds triggering environmental effects. The usual concentration of copper oxide in paints varies from 20% to 76% wt [4]. As daily the copper cost increases, the use of copper oxide in the paints makes this technology an expensive solution.
The anti-biofouling (AF) efficacy in a chlorinated rubber-based coating and two polydimethylsiloxane (PDMS)-based coatings were evaluated in a stationary field exposure assays. The evaluation included surface wettability and morphology [5]. Today, the effect of the inclusion of carbon nanotubes into the marine paints is subject of evaluation [6].
Barnacles have become a model organism to study the process of biofouling [7,8]. The trend today is to identify natural compounds to act as antifoulants [9-15]. There are several organisms acting as natural chemicals against fouling [16-19]. Several metabolites possess antimacrofouling, antifungal, and antiprotozoan properties thus having potential to be developed as antifouling agents [20-26]. The majority of them are tested in laboratory assays. There are few assays verified under natural field in paint formulations [27]. This is important for assessing their possible use as antifoulants. It is known that marine organisms show resistance to epibiosis [26], counting species of algae that contain a diverse spectrum of chemical entities. Algae seem to be chemically protected by surface-bound or continuously released water-soluble compounds that can deter invertebrate larvae from settling. Algal metabolites affect the growth behavior of settle down organisms. Separation of these bioactive metabolites may lead to the progress of new eco-friendly antifouling paints.
Besides fouling, one has to protect the metals against corrosion. In our laboratory, we developed coatings with nanocontainers including inhibitors to induce self-healing [28]. The nanocontainers are disrupted during corrosion and release the necessary compounds for the repair of the coating. We developed a number of nanocontainers (e.g. CeMo, TiO2, CuO, ZnO, etc.) that were incorporated into industrial coatings exhibiting also antimicrobial properties aside the anticorrosion properties [29,30].
This work presents a new self-healing marine paint technology with anticorrosion and antifouling properties for ships’ hulls by incorporating copper oxide nanocontainers loaded with natural antifoulants in topcoat formulations (bromosphaerol) and nanocontainers loaded with corrosion inhibitors (8Hydroxyquinoline - 8HQ) self-healing inducers in primer formulations which underwent surface modification forming nanocomposites with materials for biofouling and corrosion prevention. The CuO nanocontainers were also loaded with SeaNineTM211 and tested in the lab and open sea water via a ship hull paints.
Characterization techniques
Scanning electron microscopy (SEM) and Transmission Electron Microscopy (TEM) were employed to observe images using a FEI Inspect microscope operating at 25kV and a FEI CM20 microscope operating at 200kV, equipped with a Gatan GIF200 Energy Filter utilized for EF-TEM elemental mapping, respectively. A Solartron ModuLab® XM MTS system was used to acquire the Frequency Response Analysis (FRA) measurements. A Malvern nano series system was used to determine the size and z-potential of the nanocontainers. The samples were painted using commercial Wilkens paints in which the nanocontainers were incorporated and tested in the Mikrolimano harbor very close the Piraeus harbor. The paints included a primer, an intermediate anticorrosion paint and on the top an antifouling paint. To simulate conditions similar to moving ship with 14 knots, the samples were placed in a tube at the end of which a propeller was placed moving the water through the samples simulating a moving ship environment. The tests were conducted for about a year where samples were taken from the sea water each month for evaluation. Other samples were painted using Re-Turn paints. The test was conducted in Singapore for 35 months using a platform in the open sea water. The conditions were harsh due to warm weather the year around.
Nanocontainer production
We used analytical reagent grade chemicals like Cerium(III) acetylacetonate (Ce(acac)3, Sigma-Aldrich Chemie GmbH), Zinc acetylacetonate hydrate (Sigma-Aldrich Chemie GmbH), sodium molybdate (Na2MoO4, Panreac Quimica SA), polyvinylpyrrolidone (PVP, average molecular weight 555000, Sigma-Aldrich), potassium persulfate (KPS, Sigma-Aldrich), sodium dodecyl sulfate (SDS, Sigma-Aldrich), sodium chloride (NaCl, Sigma-Aldrich Chemie GmbH), 8-HQ (Sigma-Aldrich Chemie GmbH), Bromosphaerol (FORKYS AE Ichthyokalliergeies), SeaNineTM 211 (Dow Chemical Company) and 1-BSA (Sigma-Aldrich). The manufacturing procedures were described in our literature several times and avoid description here to avoid repetition [27,28].
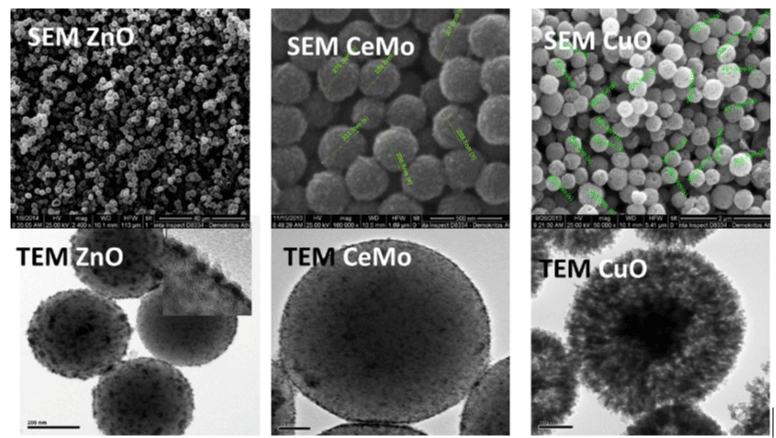
Figure 1 shows the SEM and TEM micrographs of the ZnO, CeMo and CuO nanocontainers.
Figure 1: SEM and TEM of the ZnO, CeMo and CuO nanocontainers.
One can perceive from this Figure that the nanocontainers are porous and hollow with homogeneous size distribution. One important issue is the homogenous distribution of the CuO nanocontainers in a solution before mixing with the antifouling paint. Several tests were conducted with a concentration of 1 mg/ml. In xylene the CuO nanocontainers were destroyed in less than 2 hours. In Phosphate-buffered saline (PBS), the CuO nanocontainers have very poor dispersion and form lumps when loaded with sea-nine© 211. In methanol, CuO nanocontainers achieved good dispersion and were not destroyed after stirring of three days.
Figure 2 shows the SEM micrographs of the primer and the three-layer paints consisting of the primer (~70 μm), anticorrosion (~142 μm) and antifouling (~136 μm) layers. These layers were doped by the nanocontainers except the primer coating. The anticorrosion coating was always doped with CeMo loaded with 8HQ to the total amount of 5 wt. %. The antifouling coatings were doped with the nanocontainers as shown in table 1 in a total amount of 8 wt. %.
| Table 1: Shows the paints prepared for the laboratory tests. | ||
| Code of sample | Antifouling coating | Percentage |
| 1 | CuO | 8 wt.% |
| 2 | CuO (bromosphaerol, BMSPH) | 8 wt.% + weight by BMSPH (25%) calculated from loading |
| 3 | CuO (seanine211, SN211) | 8 wt.% + SN211 weight corresponding to that of Wilkens (11.6 g/kg) |
| A | ZnO | 8 wt.% |
| B | ZnO (BMSPH) | 8 wt.% + weight of BMSPH (40%) calculated from loading |
| E | ZnO (SN-211) | 8 wt.% + SN-211 weight corresponding to that of Wilkens (11.6 g/kg) |
| I | ZnO (SN-211) + CuO (SN-211) | 4wt.% + 4wt.% + SN-211 weight corresponding to that of Wilkens (11.6 g/kg) |
| II | Ecomar AF 2000 (38wt% Cu) + SN-211 |
|
| III | Ecoloflex SPC 200 (Nippon) | |
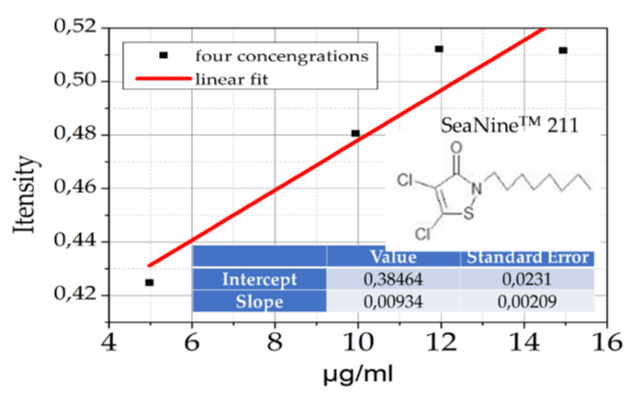
Figure 2: Reference curve of SN211 in solvent xylene with ultraviolet spectroscopy – visible.
The concentration of the compounds incorporated into the nanocontainers was determined via ultraviolet – visible spectroscopy. For example, we developed a reference curve of SN211 in solvent xylene of defined concentrations. Initially, SN211 was isolated from the commercial solution xylenol. The purity of the product was confirmed by NMR spectroscopy 1H. Figure 2 shows the reference curve for SN211 in four defined amounts dissolved in xylene.
It was observed that SN211 has very good solubility in methanol and very poor solubility in PBS. Similar work was conducted for bromosphaerol and 8-Hydroxyquinoline for the CuO, ZnO and CuO nanocontainers to determine the Encapsulation Efficiency (EE) and Loading Capacity (LC). Table 2 shows the results of this study.
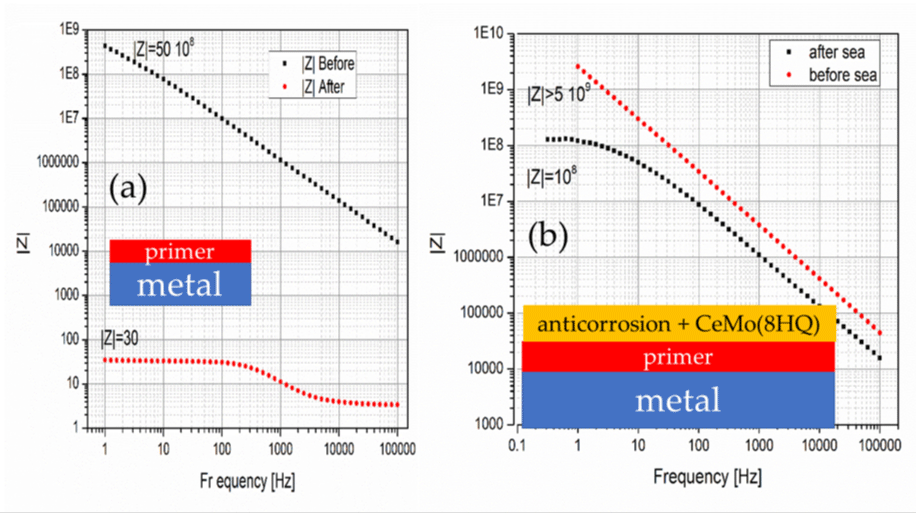
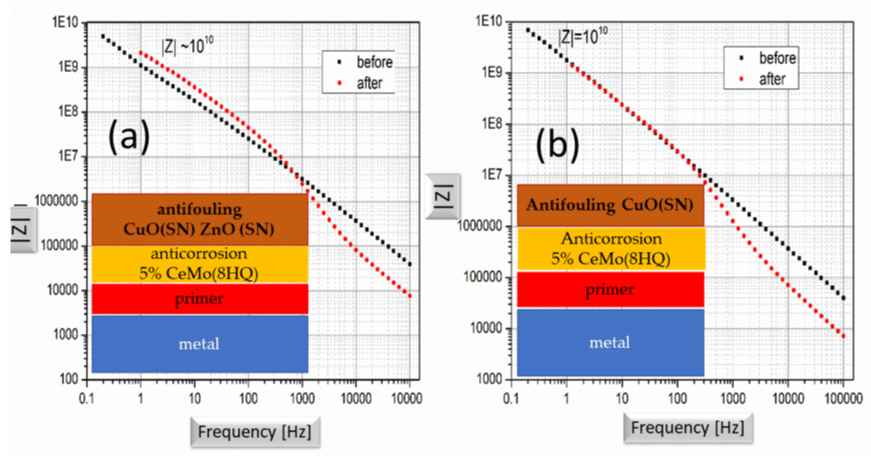
The samples were treated in seawater in the Mikrolimano harbor (close to Piraeus harbor) for three months and then were brought to the lab and were further exposed to 0.5 M NaCl solution for 48 hours. Electrochemical impedance spectroscopy (EIS) measurements were conducted in 0.5 M NaCl solution. Figure 3a shows the performance of the primer before and after exposure in the sea. Rp is ~10 108 Ω before exposure to the sea water and dropped drastically after the exposure. The primer doesn’t offer any corrosion protection to the metal. Figure 3b shows the performance of the two-layer coating consisting of the primer and the anticorrosion layer with 8 wt. % CeMo(8HQ). It is important to mention here that the paints used here were free of any additives but the only chemical added to the paints were the nanocontainers supplementary to the basic paint formulation. One can perceive from Figure 3b an improvement of Rp (>5 109 Ω) with respect to the primer protection and a drop of Rp to the value of 108 Ω, still significant protection.
Figure 3: EIS of the primer (a) and primer on top of the corrosion protection paint with CeMo(8HQ) (b).
Performance of the primer before and after exposure of the sample for three months into sea water.
Behavior of the primer plus anticorrosion paint with 8 wt. % CeMo(8HQ) before and after three months exposure to the sea water.
Figure 4 shows the paint consisting of the primer, anticorrosion with CeMo(8HQ) layer and antifouling paint with CuO (Bromosphaerol). Rp is better than 5 109 Ω for the sample before exposure to the sea and slightly dropped to the value of greater than 2 109 Ω for the sample exposed to the sea water for three months. The top layer of antifouling with CuO (Bromosphaerol) improves the corrosion protection of the marine paint with the nanocontainer addition.
Figure 4: EIS measurement of the paint consisting of a top layer (antifouling paint with CuO (Bromosphaerol), intermediate layer (anticorrosion with CeMo(8HQ) and primer before and after exposure to the sea water for three months.
Figure 5 shows the EIS of the three-layer system the top of which is doped with nanocontainers loaded with SeaNineTM 211. The one in the left (a) consists of top layer with the antifouling paint incorporating the CuO (SN) and the ZnO (SN) nanocontainers, the intermediate anticorrosion paint incorporating the CeMo(8HQ) nanocontainers and the primer. The other on the left (b) composed of the antifouling paint including the CuO (SN) nanocontainers, the in-between anticorrosion paint integrating the CeMo(8HQ) nanocontainers and the primer. Rp for the left coating without exposure to the sea water was about 1010 Ω. After exposure to the sea water for three months, Rp improves as shown by the FRA response that means better anticorrosion behaviour of the protective paint. This behaviour in the literature can be attributed to self-healing of the paint [26]. Similar behavior exhibits the paint of Figure 5b with the top layer consisting of antifouling paint with CuO (SN) nanocontainers.
Figure 5: EIS of the paints: a) top layer the antifouling paint incorporating the CuO (SN) and the ZnO (SN) nanocontainers, intermediate anticorrosion paint incorporating the CeMo(8HQ) nanocontainers and primer b) top layer the antifouling paint including the CuO (SN) nanocontainers, in-between anticorrosion paint integrating the CeMo(8HQ) nanocontainers and primer.
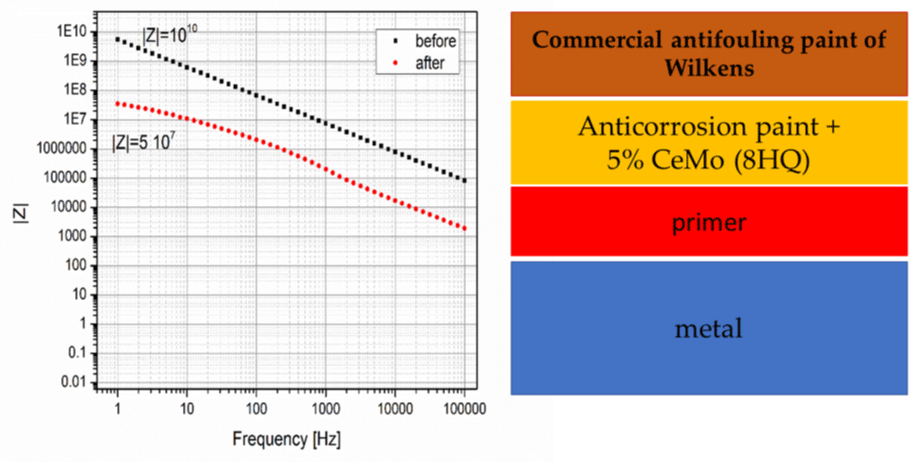
Figure 6 shows the EIS paint consisting of the commercial paint of Wilkens, the anticorrosion paint with the CeMo (8HQ) nanocontainers and the primer. The paint before exposure has Rp about 1010 Ω but drops to the value of about 5 107 Ω after exposure to sea water for three months.
Figure 6: EIS of the paint consisting of the commercial paint of Wilkens, the anticorrosion paint with the CeMo (8HQ) nanocontainers and the primer before (black) and after (red) exposure to sea water.
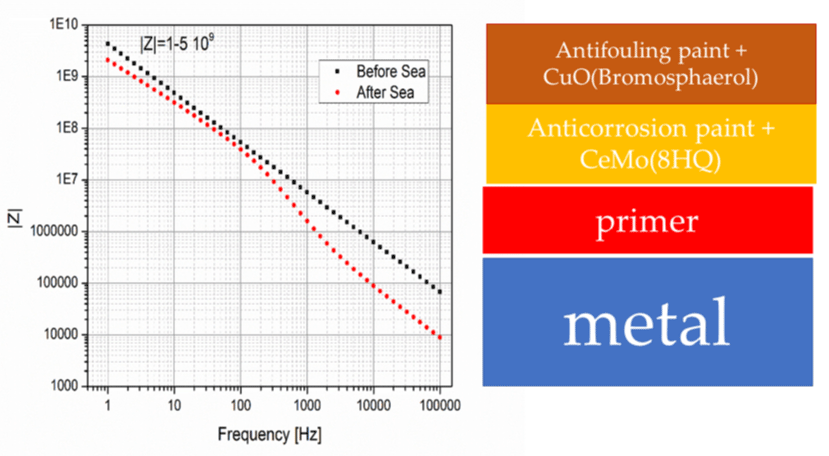
The question is whether the new technology exhibits good antifouling properties. This was answered by both paint technologies of Wilkens and Re-Turn in two different conditions the one in Greek sea waters and the other in the harsher Singapore sea water environment due to the warmer weather conditions. An extensive study will give a complete analysis but it is important to show here the excellent results we observed from the exposure of the Re-Turn paints using CuO (SN211) on the top paints for thirty-five months. Figure 7 shows the results of this work. Fouling is composed of sediments, slime and green algae. There were no other foulers.
Figure 7: Exposure of the Re-Turn antifouling with 4 wt. %CuO (SeaNineTM 211) and anticorrosion paint in the Singapore sea waters.
It is important to note hear that another benefit of our nanotechnology was the increase of the contact angle of the sea water with our paint system was 110o as compared to 77o measured in the commercial paints of Re-Turn. This has important implications in the reduction of drug resistance of the ship in the sea increasing the speed of the ship, reducing petrol consumption and reducing the air pollution.
The technology developed here was also tested by painting fraction of two ship hulls. The one ship called Sea Anemos was a commercial passenger ship traveling with 14 knots between Ancona (Italy) and Patras (Greece) in the Adriatic Sea for one year. That ship was painted in a front stripe with the Wilkens paints including our technology. The other ship was called Berge Arzew in Norway traveling for more than one year in Nord Sea and was painted in the front using the Re-Turn paints incorporating our nanotechnology. Figure 8 show the results of the tests. One can observe from this Figure negligent fouling in the section we painted with the nanotechnology.
Figure 8: Show the results of the tests. One can observe from this figure negligent fouling in the section we painted with the nanotechnology.
The current ship paint technology is using copper oxide in amounts between 26 to 76 wt. %. Here, 5 wt. % CuO nanocontainers is sufficient to obtain even better results, leading to a dramatic reduction of cupper in the paints resulting in less pollution due to cupper. The use of Bromosphaerol is important achievement because it is a natural product compatible with the sea environment, so there is need to get permission to use it in the paints. The self-healing ability of the improved coating due to the incorporation of the nanocontainers is another major improvement of the current technology. The increase of the contact angle via surface modifications due to nanocontainer incorporation offers another advantage of our nanotechnology. This successful application growing expectations of nanotechnology to bring new results for the improvement of existing products.
The author thanks the Greek General Secretary for Science and Technology and the European Union via the FP7 program MUST (Multi-level protection of materials for vehicles by ‘smart’ nanocontainers) for funding this work. He also thanks the WILKENS, Re-Turn and FORKYS companies for providing the chemicals.
- Armstrong E, Boyd KG, Burgess JG. Biotechnology Annual Review. 2000; 6: 221-241.
- Schultz MP. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling. 2007; 23: 331-341. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17852068
- Townsin RL. The ship hull fouling penalty. Biofouling. 2003; 19: 9-15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14618699
- Brooks NS, Waldock M, Hellio C, Yebra D, Woodhead Publishing Ltd. Cambridge. 2009; 495.
- Lang Y, Sun Y, Yu M, Ji Y, Wang L, et al. Differential Colonization Dynamics of Marine Biofilm-Forming Eukaryotic Microbes on Different Protective Coating Materials. Polymers. 2019; 11: 161. PubMed: https://www.ncbi.nlm.nih.gov/m/pubmed/30960145/#fft
- Ji Y, Lang Y, Wang L, Liu B, Zhang Z. Int. Biodeterioration & Biodegradation. 2018; 129: 195-201.
- Walker G, Yule AB. J Mar Biol. Ass. UK. 1948; 64: 429.
- Dreanno C, Kirby RR, Clare AS. Locating the barnacle settlement pheromone: spatial and ontogenetic expression of the settlement-inducing protein complex of Balanus Amphitrite. Proc Biol Sci. 2006; 273: 2721- 2728. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1635498/
- Pawlik J. Oceanogr. Mar Biol Ann Rev. 1992; 30: 273.
- Abarzua S, Jakubowski S, Eckert S, FP. Bot Mar. 1999; 42: 459.
- Rittschof D. In Marine chemical ecology. Marine science series. J B McClintock, BJ Baker, CRC, London. 2001: 543-566.
- de Nys R, Steinberg PD. Curr Opin Biotechnol. 2002; 13: 244.
- Burgess JG, et al. Biofouling. 2003; 19: 197.
- Ralston E, Swain G. Bioinsp Biomim. 2009; 4: 1.
- Qian PY, Xu Y, Fusetani N. Biofouling. 26: 223.
- Engel S, Jensen PR, Fenical W. J Chem Ecol.2002; 28: 1971.
- Hellio C, Pascal Berge J, Beaupoil C, Le Gal Y, Bougougnon N. Biofouling. 2002; 18: 205.
- Paul VJ, Puglisi MP. Nat Prod Rep. 2004; 21: 189.
- Hellio C, Bourgougnon N, Le Gal Y. Biofouling. 2000; 16: 235.
- Steinberg PD, de Nys R. J Phycol. 2002; 38: 621.
- da Gama BA, Pereira RC, Carvalho AGV, Coutinho R, Yoneshigue-Valentin Y. Biofouling. 2002; 18: 13.
- Faimali M, Sepcic K, Turk T, Geraci S. Biofouling. 2003; 19: 47.
- Lane L, Nyadong L, Galhena AS, Shearer TL, Stout EP, et al. Proc Natl Acad Sci. USA. 2003; 100: 6916.
- Tsoukatou HM, Maréchal JP, Aldred N, Beaupoil C, Clare AS. Mar Biotechnol. 2005; 7: 297.
- Fusetani N. Biofouling and antifouling. Nat Prod Rep. 2004; 21: 94104. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15039837
- Ali MS, Saleem M, Yamdagni R, Ali MA. Nat Prod Rep. 2002; 16: 407.
- Briand JF. Marine antifouling laboratory bioassays: an overview of their diversity. Biofouling. 2009; 25: 297-311. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19191083
- Kordas EK, Efthimiou EK. The Sole-Gel Handbook. Self-Healing Coatings for Corrosion Protection of Metals, Volume 3: Application of Sol-Gel Materials. Eds. David Levy and Marcos Zayat, Wiley-VCH, Verlag GmBH & Co. 2015; 44: 1371.
- Kartsonakis IA, Athanasopoulou E, Snihirova D, Martins B, Koklioti MA, et al. Corrosion Science. 2014; 85: 147-159.
- Kartsonakis IA, Balaskas AC, Koumoulos EP, Charitidis CA, Kordas G. Progress in Organic Coatings. 2013; 76: 459– 470.